Featured Posts

8 Reasons Why Many Patients Prefer Dental Implants
The implant can restore your smile to its good health and strength. From being comfortable to making one feel confident, some positive aspects of these implants can make a big difference in how you laugh or think about yourself. This is why most patients would […]
health
What is a Personal Injury Demand Letter?
In Personal injury, you can come across a special demand letter. It is similar to a letter you write when you have been injured or need assistance. Here is all about demand letters and why they are crucial, particularly when someone has been hurt in […]
Law & Legal
6 Ways Meditation Helps Improve Physiotherapy Outcomes
Have you ever heard of a situation where meditation has benefited people who are sick and need a quick recovery? MVA physiotherapist also confirms that meditation may be used to attain pain reduction and reduce anxiety levels. Moreover, it has additional benefits in bringing one’s […]
health



8 Reasons Why Many Patients Prefer Dental Implants
The implant can restore your smile to its good health and strength. From being comfortable to making one feel confident, some positive aspects of these implants can make a big difference in how you laugh or think about yourself. This is why most patients would […]
healthThe implant can restore your smile to its good health and strength. From being comfortable to making one feel confident, some positive aspects of these implants can make a big difference in how you laugh or think about yourself. This is why most patients would go for dental implants instead of other procedures.
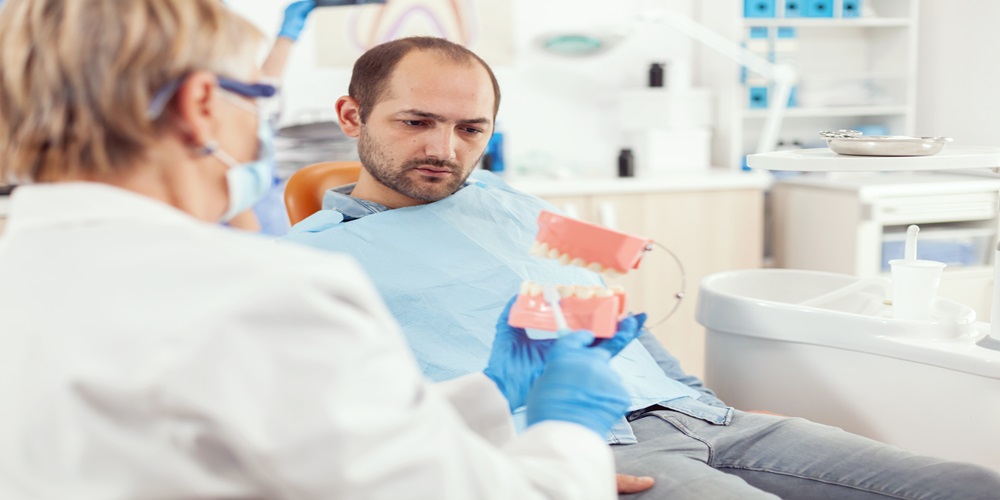
1. They Look and Feel Natural
When you have a missing tooth, an implant usually looks so natural that no one will know. Eating or speaking feels like it is your teeth. So, you can always smile and chew without worrying about being unfamiliar or different. This, therefore, means that with the help of implants, you can get a natural smiling face.
2. Sturdy and Long-lasting
These dental implants could last for several years. This makes them ideal for people with sensitive teeth or wanting long-term tooth replacement. This means they dot need to be frequently replaced by anyone using them, giving them more time to enjoy their smiles without worrying about their teeth health.
3. Better Oral Health
Good oral health requires maintaining strong, healthy teeth and gums, so dental services involve replacing missing teeth and ensuring that jawbones remain firm through dentures. Dental implants prevent the shrinking or weakening of the jawbone after losing its tooth. But when left unattended, it shrinks because the tooth is gone, resulting in loss of bone from the jaw structure.
4. Improved Comfort
Occasionally, dentures may not fit perfectly, especially if they rub against your gum, causing discomfort. However, unlike them, dental implants are like mouth’s custom-made shoes. They fit correctly, and no pain or irritation is experienced while eating, talking, and laughing. With Implants, you can speak, eat, and smile without discomfort due to denture-related problems.
5. Ease of Use and Functionality
With dental implants, you can enjoy all your preferred meals. You can talk to your friends and brush your teeth as usual. They fit so well and work so smoothly that you will not remember even having them on. This means that you can eat without extra effort, have excellent talks, and keep your teeth clean; all these are natural processes. With dental implantation, everything comes naturally.
6. Stability and Assurance
Once they are in place, they offer the wearer a firm fit that does not come loose or move around, providing stability. Therefore, eating or talking will be done without fear of losing one’s tooth.
7. Preserves Teeth Adjacent To The Missing One
Some treatments for replacing lost teeth may require modifying or removing the nearby ones to accommodate the new implant. There is no need to change any other tooth because an implant fits perfectly well within the gap left after extraction. This preserves their original looks and helps maintain a smile appearance. So even if the implant is still in place, other existing teeth remain unaffected.
8. Confidence Boosting
This new tooth fits seamlessly into your mouth and restores the original look of your smile. When you feel like your teeth look great, it rebuilds your self-esteem naturally. Therefore, this elevated confidence level makes one’s smile radiant and brightens their day.
Conclusion
Most patients highly favour dental implants since they come with many advantages that range from looking good and feeling right to lasting longer than other alternatives. They provide comfort, stability, and better oral health. They also restore self-image by boosting confidence by giving back a complete smile. For consultation and expert advice on dental implants, please contact us.

What is a Personal Injury Demand Letter?
In Personal injury, you can come across a special demand letter. It is similar to a letter you write when you have been injured or need assistance. Here is all about demand letters and why they are crucial, particularly when someone has been hurt in […]
Law & LegalIn Personal injury, you can come across a special demand letter. It is similar to a letter you write when you have been injured or need assistance. Here is all about demand letters and why they are crucial, particularly when someone has been hurt in an accident.

Definition of a Personal Injury Demand Letter
A demand letter about personal injury contains information on how one was injured, what happened and the amount requested as compensation.
Purpose of a Demand Letter
Demand letters aim to ask for money from those who caused injuries. These letters are meant to show why they owe the payment for damages. The sums such victims require are also included in this kind of correspondence, as they should be able to cover medical and other related expenses.
Components of a Personal Injury Demand Letter
Introduction
The introduction part of personal injury cases serves as an identifier. In addition, it informs the reader about your intentions behind writing the letter.
Statement Of Facts
In your demand letters statement of facts section, you can explain all events leading up to the accident or incident that caused your injury. You may also supply proof or prove negligence in slip and fall accidents or any other incidents precipitated by those addressed here.
Liability And Damages
Liability refers to who caused the harm, while damages refer to what bad things happened because the injury was caused. Hence, this part explains why the party you are writing to is accountable for your injuries and how these injuries have affected you.
Medical Treatment And Expenses
In this section, you should discuss the care you need due to this injury. You will also mention the doctors who treated it and the money you had to pay for medical bills.
Lost Wages
Lost wages are the earnings that a person fails because they cannot go to work due to their injury. This part explains how much income loss resulted from your injuries and why someone should make payment for this particular form of compensation.
Pain And Suffering
These terms refer to physical and emotional pain caused by your injury. In this paragraph, you describe the suffering, pain and other difficulties which have been brought about by your accident.
Demand for Damages
This is the part that shows how much money you want. It should be enough to cover your hospital bills, any lost chances of doing business, pain, and other things that have resulted from the accident.
Deadline for Response
The deadline for response is the day by which you expect the person you are writing to act as stated in the demand letter and reply to the sender of this request. It allows them some time within which they must answer and signifies your seriousness in pursuing compensation for injuries suffered during an accident.
Critical Issues To Consider When Writing A Demand Letter
Clarity And Brevity
Avoid using convoluted terms and phrases when drafting a demand letter since they make it hard for a reader to know precisely what you need and why. Keep your letter short but concise so that people can understand what you mean.
Documentation and Evidence
This must be considered; evidence supporting your claims is crucial when writing a demand letter. These may include but are not limited to medical records, receipts, and pictures of injuries sustained. Soundproof can boost one’s claim, thus making one’s demands more persuasive.
Professional Tone
This type of communication means always being respectful in one’s written appeal. The language shall be brief so that the script will use restrained vocabulary.
Specificity in Damages
Describe what expenses you have incurred due to the harm done to your body in exact terms like hospital bills and loss of work. Thereby making both sides aware of each other’s needs when communicating.
Legal Basis for Claim
When writing a demand letter, you must know why you are entitled to compensation in law. Therefore, you will have to prove that the individual you are addressing is liable for your injuries and should be made to pay you.
Conclusion
Demand letters can greatly help after any accident. Please keep it simple and friendly and list the specifics when drafting one of these letters. It may look like narrating your story and requesting assistance politely. Feel free to ask to a Injury lawsuit attorney about writing a demand letter or more on how personal injury cases can be handled easily.

6 Ways Meditation Helps Improve Physiotherapy Outcomes
Have you ever heard of a situation where meditation has benefited people who are sick and need a quick recovery? MVA physiotherapist also confirms that meditation may be used to attain pain reduction and reduce anxiety levels. Moreover, it has additional benefits in bringing one’s […]
healthHave you ever heard of a situation where meditation has benefited people who are sick and need a quick recovery? MVA physiotherapist also confirms that meditation may be used to attain pain reduction and reduce anxiety levels. Moreover, it has additional benefits in bringing one’s mind and body together more effectively. Here is the role of meditation in our health and recovery.

- Reduction of Stress and Anxiety
Meditation trains you to center your mind while relaxing your body. With regular practice, you can learn better ways of managing stress or coping with anxiety.
Patients who engage in their exercises during physical therapy sessions have low stress levels. This means that with less stress, they can concentrate well on their movements. This can help them gain maximum advantages from any given treatment process.
- Enhanced Pain Management
Meditation teaches individuals how to be present, focus on the mind, and relax their bodies.
They can do this and calm down over-stimulated nerves when experiencing pain. Practicing meditation regularly will enable you not only to cope with pain but also make it feel less severe.
- Enhanced Focus and Concentration
Meditation helps individuals to get more control of their minds and concentrate on what they are doing now.
As a result, these distractions will not tamper with an individual’s performance level. In physiotherapy, focus is vital when performing exercises. Patients gain maximum advantage from their therapy because it enables them to carry out movements correctly.
- Promotion of Mind-Body Connection
Your body can react due to how you feel mentally, affecting your physical body since the two are linked. Meditation helps people establish that bond between their minds and bodies by allowing them to be more in touch with themselves.
Meditation is about helping individuals understand why their bodies may feel this way. This is because it focuses on knowing more about our bodies’ daily stimuli. This makes an individual more aware of how his/her emotions can affect him/her physically.
- Boosting Recovery and Rehabilitation
To heal faster after an injury or illness, you have to think about ways to help meditate, which can help to increase the recovery process. It is a different way of relaxing your mind and body, supporting quick healing. Therefore, meditation can promote relaxation and lower stress levels, facilitating natural healing.
- Improved Mental Health
When your mind is well, it may promote your body’s healing process. Meditation is like a special kind of calming thought and feeling tranquil inside you. Meditation assists people to loosen up and eliminate stress from their brains.
Your sound mental health is connected with how well your body heals itself. A person’s body tends to recover faster from injuries or surgeries when feeling happy and less stressed.
Conclusion
Through meditation in his sessions, a therapist may increase the concentration ability of an individual patient. This helps increase mind-body connection and improves either self-confidence or self-esteem. It also helps accelerate recovery and improve the quality and quantity of sleep duration. Contact us now for professional physiotherapy advice on integrating meditation techniques into the physiotherapy program.

Conscious Living: Understanding the Environmental Impact of Garage Door Choices
In our quest for a more sustainable and eco-friendly lifestyle, it’s crucial to examine every aspect of our living spaces, including the often-overlooked garage doors. These seemingly mundane elements play a significant role in our environmental footprint. As conscientious citizens of Canada, understanding the impact of […]
Home ImprovementIn our quest for a more sustainable and eco-friendly lifestyle, it’s crucial to examine every aspect of our living spaces, including the often-overlooked garage doors. These seemingly mundane elements play a significant role in our environmental footprint. As conscientious citizens of Canada, understanding the impact of our choices is essential. This article delves into the environmental considerations associated with garage door choices, shedding light on the importance of conscious decision-making.

The Environmental Footprint of Garage Doors:
1. Material Matters:
Garage doors come in various materials, each with its own environmental implications.
– Wood: Often favoured for its aesthetic appeal, wood garage doors contribute to deforestation.
– Steel: While durable, the production of steel involves significant energy consumption and emissions.
– Aluminum: Lightweight and corrosion-resistant, aluminum is energy-intensive to extract and process.
2. Energy Efficiency:
The energy efficiency of garage doors impacts both the environment and household expenses.
– Insulation: Opting for insulated doors helps regulate temperature, reducing the need for heating or cooling.
– Weather Stripping: Proper sealing prevents drafts, further enhancing energy efficiency.
3. Manufacturing Processes:
The methods used by custom garage door manufacturers greatly influence their environmental impact.
– Local Production: Choosing locally manufactured doors reduces transportation-related emissions.
– Sustainable Practices: Manufacturers employing eco-friendly processes and materials contribute to a greener industry.
The Impact on Canada’s Ecosystem:
Canada, with its diverse ecosystems and commitment to environmental conservation, requires a thoughtful approach to sustainable living. Here’s how garage door choices can affect the Canadian landscape:
1. Climate Considerations:
– In colder regions like Alberta and Quebec, insulated garage doors become crucial for energy conservation.
– Coastal areas like British Columbia may benefit from corrosion-resistant materials due to the moist climate.
2. Wildlife and Biodiversity:
– Habitat destruction linked to certain garage door materials can impact local wildlife.
– Eco-friendly manufacturing practices contribute to the preservation of Canada’s rich biodiversity.
3. Resource Management:
– Choosing materials with minimal environmental impact aligns with Canada’s commitment to responsible resource management.
Making Informed Choices:
1. Research and Awareness:
Before making a decision, consumers should research the environmental aspects of different garage door materials.
2. Local Regulations:
Understanding and adhering to local environmental regulations ensures that choices align with community sustainability goals.
3. Lifecycle Considerations:
Assessing the entire lifecycle of garage doors, from production to disposal, provides a holistic view of their environmental impact.
Conclusion:
In conclusion, the environmental impact of garage door choices is a crucial aspect of conscious living in Canada. By considering the materials, energy efficiency, manufacturing processes, and local implications, we can make choices that align with our commitment to sustainability.
As consumers, our choices matter. Opting for garage doors from responsible and eco-conscious custom garage door manufacturers ensures that we contribute positively to the environment. Let’s take a step towards a greener future by making informed decisions. Explore the range of eco-friendly garage door options available and play your part in building a sustainable Canada.
Remember, every small decision adds up, and together we can create a more environmentally conscious and responsible living space.

8 Ways to boost the benefits of physiotherapy
Physiotherapy plays an important role in our body. It helps people overcome injuries. It manages chronic health conditions, too. Physiotherapy improves overall physical function. While receiving this treatment, there are ways to boost the benefits. Following certain steps can maximize the benefits of physiotherapy. 1. […]
healthPhysiotherapy plays an important role in our body. It helps people overcome injuries. It manages chronic health conditions, too. Physiotherapy improves overall physical function. While receiving this treatment, there are ways to boost the benefits. Following certain steps can maximize the benefits of physiotherapy.
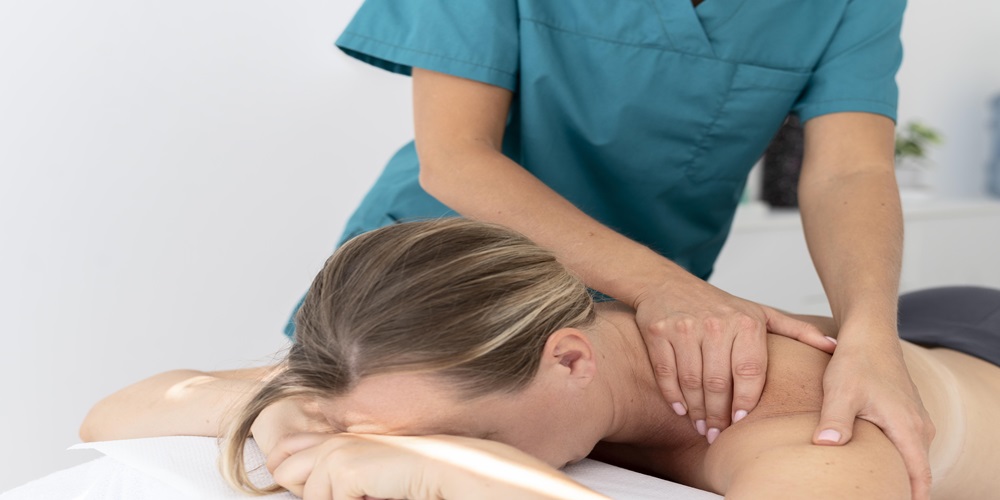
1. Talk Openly with Your Physiotherapist
You should tell them about your personalized physiotherapy programs. Discuss any discomfort or concerns you have. This open communication is vital. It ensures the treatment plan is tailored for you. The plan will address your specific needs and goals.
2. Follow the Exercise Program Strictly
Doing exercises exactly as prescribed is very important. Being consistent with the program is key. Not following it can prevent full recovery. It may lead to setbacks in your progress.
3. Stay Active Daily
Therapy sessions are very helpful. But, they are not enough. Staying active daily also aids recovery progress. Simple activities like walking or swimming can help. You could do light exercises recommended by your physiotherapist. Any extra physical activity boosts the benefits.
4. Mind Your Posture and Body Mechanics
Proper posture is emphasized in physiotherapy. Using good body mechanics helps too. This reduces strain on your muscles and joints. Poor form can worsen issues or prevent healing. Good posture prevents further injury as well.
5. Manage Stress and Get Enough Rest
Increased stress and lack of rest hinder healing. Too much stress creates tension in the body. Not resting makes it harder to recover.
6. Eat a Balanced, Nutritious Diet
You should eat foods with lean protein. Good options are fish and eggs. Eat plenty of fruits and vegetables too. Also add nuts and healthy fats. Include whole grains in your diet. Eating this way provides energy for tissue repair. The right nutrition fuels the healing.
7. Stay Well Hydrated
Hydration is absolutely vital for recovery. Water consumption is beneficial in numerous ways. Toxins are eliminated from your body via water. It carries vital nutrients to the cells.
Water lubricates the joints as well. All this supports healing and physiotherapy progress.
8. Keep a Positive Mindset
Your mindset impacts healing significantly. Having a good, positive attitude helps a lot. You should celebrate small wins along the way. Remain motivated throughout the treatment process. Mental resilience aids recovery progress tremendously.
To summarize the key points:
- Open communication with your physiotherapist is crucial
- You must follow the exercise program strictly
- Staying physically active daily aids in recovery
- Maintaining proper posture is important
- Managing stress and getting enough rest is vital
- Eating a balanced, nutritious diet provides fuel
- Staying well hydrated supports healing
- Keeping a positive mindset makes a big difference
Conclusion
Follow these eight tips. They can maximize the benefits of physiotherapy. You will heal faster and more completely. Your recovery will be aided tremendously. Using these strategies helps you reach peak health. Engage fully in the process for the best results. Collaborate closely with your physiotherapist as well. Doing so unlocks the full potential of your treatment. Our team at Active Physio Wellness Centre has extensive training in individualized physical therapy. Make an appointment right now to start along the path to a better, more functional version of yourself.

5 most typical garage door color options
The correct color for your garage door dramatically differs from your home’s look. Here are the five colors people like the most for garage doors or industrial sectional overhead doors. We’ll keep it simple and straightforward to help you choose the best color for your […]
Home ImprovementThe correct color for your garage door dramatically differs from your home’s look. Here are the five colors people like the most for garage doors or industrial sectional overhead doors. We’ll keep it simple and straightforward to help you choose the best color for your home’s outside.

White
Lots of people go for white when it comes to garage doors. It’s popular because it looks clean and brings a positive vibe. If your house has white trim, having a white garage door fits in nicely. White garage doors also reflect light, making them suitable for different homes. This light-reflecting quality adds to how nice it looks and brightens your garage area with natural light. So, white garage doors are versatile and work well for many different home styles, giving your place a bright and welcoming feel.
Neutral Colors (Beige, Tan)
Many homeowners like neutral colors like beige and tan. These colors work well with many colors you might have outside your house. Beige or tan is a safe bet, giving your garage door a classic and timeless look. Whether your home looks more traditional or modern, these neutral tones fit in smoothly, adding a simple yet elegant touch. Beige and tan colors can stand the test of time, staying stylish for a long while. That’s why they are good choices for people who want a garage door color that stays cool over the years.
Gray
Soft grey is a stylish and classy pick for garage doors. Many people like it because it looks chic. Warm grey goes well with almost any color and gives a welcoming vibe. It’s especially great for houses with wood siding on the outside. The warm grey with wood becomes a cosy, inviting appearance for the garage door. It works to make your home look even better for people looking.
Black
Black is a classic color for garage doors. This has a very timeless and classy feel. While black absorbs very little light and looks dark, it is still used. When discussing black, it is essential to understand the amount of natural light around your garage. With enough light, the black can provide your home’s exterior with a little hint of elegance.
Wood-Look Finishes
Stained wood-look steel doors are an excellent option for those who love the natural look of wood but want something other than the upkeep. These doors feature wood tones ranging from medium oak to walnut. A wood-look finish can create a cosy and welcoming vibe outside your home. It is a very convenient option for those who love the beauty of the wood without dealing with the many inconveniences associated with maintaining it. Contact one of the popular garage door builders in Canada to inquire about their maintenance contract.
Conclusion
These garage door color options provide a wide range of choices that can fit into various house types and personal tastes. Whether you prefer the sophistication of the grey, the classic beauty of the black, or even wood-look styles for a touch of warmth, each choice has its charm. Through such color palettes, homeowners can increase the curb appeal of their houses with a lasting and very positive impact.

by Liam Snow
7 Ways Exercise can boost brain function
When we talk about getting healthier, we often forget how exercise can also make our brains work better. Here are seven ways exercise helps our brain to be sharper and work well according to online top-notch fitness programs. 1. Memory and Learning Exercise is more […]
FitnessWhen we talk about getting healthier, we often forget how exercise can also make our brains work better. Here are seven ways exercise helps our brain to be sharper and work well according to online top-notch fitness programs.

1. Memory and Learning
Exercise is more than just sweating; it helps our memory and learning. When we exercise, our brain releases growth factors. These growth factors help to make our brain cells healthier. They also help in creating new blood vessels. Studies show that regular aerobic exercise makes the hippocampus more better. This is the part that helps in learning and memory.
2. Reduction of Anxiety and Depression
It makes the body comfortable and our brain alert. It reduces your stress and the likelihood of being depressed. Exercise also makes us sleep better and is suitable for our mental health. It helps us handle stress and worry, which are big reasons our thinking can get fuzzy.
3. Fights against Cognitive Decline and Dementia
As we get older, we can about our thinking getting worse, leading to dementia. Doing regular exercise can help prevent this. Research shows that people who don’t move much are almost twice as likely to have their thinking decline. This means that moving our bodies can lower the chances of our thinking worsening as we age.
4. Neurogenesis
Exercise does something unique for our brains by helping create new brain cells. This process is what is called neurogenesis. Making new brain cells is crucial for improving our thinking—helping us learn, think, and remember things more easily. This is why exercising at any chance you get is essential. This will help you in the long term.
5. Expression of Genes and Neurotransmitters
Exercise helps boost the activity of specific genes that help protect our brains. These genes meet the need for antioxidants to stimulate our brains. Exercise also affects dopamine, noradrenaline, and serotonin – neurotransmitters. These have significant roles in how our brains function.
6. Protection Against Aging Fast
Getting older can make our brains less sharp, and exercise can help. It protects our brains from getting old and some diseases. Exercise also connects our brain cells healthily. This is a way of assisting them to talk to each other better.
7. Vascularization and Antioxidation
Exercise assists blood vessels that are compatible with the brain. It also functions as an antioxidant that preserves the integrity of our neurons. Plus, exercise helps control particular factors that help our brain cells grow and stay healthy. When you combine most of these things, it makes our brains work better overall.
Conclusion
Exercise is a powerful tool that keeps our brains in shape. It works by making new brain cells to protect against aging. The benefits of exercise on our cognitive function are something you can see. It is essential that you exercise daily. Consistency will help you build this resilience that can also help you get the brain benefits. Keep doing the exercises for a healthier body and a sharper mind. Get assistance for more tips and guidance on staying healthy.

Shoplifting in Canada: Understanding Criminal Charges
Shoplifting is a prevalent issue in Canada, and it’s essential to understand the criminal charges and legal consequences that come with this offence. This article aims to provide an informative and educational guide for our Canadian readers, shedding light on the seriousness of shoplifting, its […]
Law & LegalShoplifting is a prevalent issue in Canada, and it’s essential to understand the criminal charges and legal consequences that come with this offence.
This article aims to provide an informative and educational guide for our Canadian readers, shedding light on the seriousness of shoplifting, its legal ramifications, and the importance of seeking professional help when facing such charges.
I. What is Shoplifting?
Shoplifting, also known as theft under the Criminal Code of Canada, refers to the act of taking or attempting to take something from a retail store without paying for it. Shoplifting can take various forms, including:
- Concealing merchandise on one’s person or in bags.
- Swapping price tags to pay a lower price.
- Intentionally damaging goods with the intent not to purchase them.
- Collaborating with an employee to commit theft.
II. Legal Consequences of Shoplifting in Canada
Shoplifting is not a minor offence in Canada. The legal consequences can be severe, depending on the value of the stolen goods and other circumstances.
Here are the primary legal consequences of shoplifting:
- Criminal Charges: Shoplifting is considered a criminal offence in Canada, and those caught may face charges under the Criminal Code. These charges can have a lasting impact on one’s criminal record.
- Penalties: Penalties for shoplifting can include fines, probation, community service, or even imprisonment. The severity of penalties depends on the value of the stolen goods, prior convictions, and the circumstances of the offence.
- Criminal Record: A shoplifting conviction can result in a permanent criminal record, which can have significant consequences on future employment, travel opportunities, and personal reputation.
- Civil Liability: In addition to criminal charges, shoplifters can be held civilly liable for damages, meaning they may have to pay restitution to the retailer for the stolen goods and any related losses.
- Employment Implications: Having a shoplifting conviction on your record may limit future job opportunities, as many employers conduct background checks. It’s crucial to seek legal counsel to understand your rights and options.
III. Why You Need Professional Legal Help
Facing shoplifting charges can be daunting, and that’s where affordable criminal lawyers in Brampton can make a significant difference.
Here’s why you should consider seeking professional legal assistance:
- Legal Expertise: Criminal lawyers in Brampton have the knowledge and experience to navigate the legal system effectively, ensuring that your rights are protected and advocating for the best possible outcome.
- Reducing Charges: A skilled attorney can work to reduce or dismiss your charges, minimizing the impact on your criminal record.
- Sentence Mitigation: If you are convicted, a lawyer can help negotiate for reduced penalties, such as fines or community service, instead of imprisonment.
- Guidance and Support: Legal professionals can provide guidance and support throughout the legal process, ensuring that you make informed decisions about your case.
Conclusion
Shoplifting in Canada is a serious offence with significant legal consequences, potentially affecting your future in profound ways. To protect your rights and ensure the best possible outcome, it’s crucial to seek the assistance of professional criminal lawyers in Brampton.
If you’re facing shoplifting charges or want to learn more about your legal options, don’t hesitate to reach out to our experienced team of lawyers. We are here to provide the support and expertise you need during this challenging time.
Remember, when it comes to shoplifting charges in Canada, the right legal representation can make all the difference in securing a more favourable outcome for your case. Don’t face this legal battle alone; take action now to protect your future.

Avoid Making During Your Home Renovation
Undertaking a home renovation is exciting but can also be fraught with challenges. To help ensure your project’s success, here are seven common mistakes to steer clear of: 1. Setting an Unrealistic Budget It is a common pitfall to underestimate the cost of a home […]
Home ImprovementUndertaking a home renovation is exciting but can also be fraught with challenges. To help ensure your project’s success, here are seven common mistakes to steer clear of:

1. Setting an Unrealistic Budget
It is a common pitfall to underestimate the cost of a home renovation. To avoid this mistake, ensure your budget includes a contingency fund of about 20%. The buffer is for unexpected renovation expenses. This includes structural problems, surprise repairs, or design changes. If you prepare for unforeseen events, you’ll be ready for any unexpected financial surprises.
2. Not Complementing the Original Architectural Style:
When you add on or update your home, keeping the original style and adding new elements is important. Your addition should harmoniously complement the existing style. If you use this method, your home will look the same and be worth more when you sell it. Combining old and new elements makes a beautiful and seamless living space.
3. Sacrificing Function for Form
A beautiful renovation is desirable, but it should also serve practical purposes. Before finalizing your design, consider how you’ll use the space daily. When you design your home, consider where you want doors, windows, outlets, and foot traffic. If you focus on form and function, your renovated space will improve your life and meet your needs.
4. Being Too Trendy
While trendy designs can be tempting, they often have a limited shelf life. It’s essential to balance incorporating fresh, modern elements and maintaining timeless design principles. To keep your renovation looking good for a long time, focus on design choices that will stay in style. This method helps you get the most out of your investment and keeps your home looking good for a long time.
5. Rushing the Job
Impatience during a renovation project can lead to costly mistakes and subpar results. Resist the urge to rush through the process. Instead, allocate sufficient time for thorough planning, proper execution, and necessary inspections. By renovating at a steady pace, we can ensure that each phase is done well.
6. Neglecting the Budget
It’s important to make a budget and stick to it while working on the project. To stay within your budget, don’t buy things you don’t need or make sudden decisions that cause you to spend too much. Manage your budget well, track expenses, prioritize spending, and make informed choices. Taking care of your budget helps you stay financially secure and maximize your money.
7. Neglecting the Team Dynamic
A successful home renovation often involves collaborating with home remodeling experts, contractors, designers, and tradespeople. Ensure that everyone involved understands your vision, project goals, and timelines. To keep your renovation on track, stay updated, and have clear expectations. This helps maintain a cohesive team and allows for better understanding.
Conclusion
You can confidently begin renovating your home by following these tips and avoiding mistakes. To have a successful renovation, start by planning carefully. Create a realistic budget and balance form and function. Prioritize timeless design principles. This will ensure your vision and your home’s future are well-invested in.
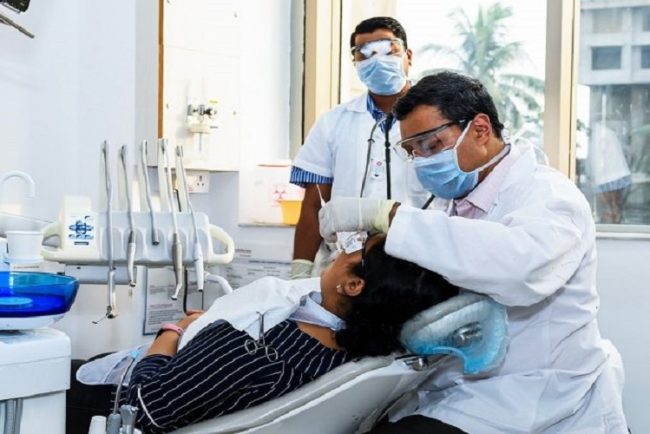
When should you see a dentist for your wisdom teeth?
Sometimes, people may have problems dealing with their wisdom teeth. According to family dentistry, many people don’t know how to care for wisdom teeth. Here are simple signs indicating it’s time to see a dentist for your wisdom teeth. Pain and Discomfort Some discomfort or […]
healthSometimes, people may have problems dealing with their wisdom teeth. According to family dentistry, many people don’t know how to care for wisdom teeth. Here are simple signs indicating it’s time to see a dentist for your wisdom teeth.
Pain and Discomfort
- Pain and Discomfort
Some discomfort or pain in your back teeth can signify some issues with your wisdom teeth. Many people usually have this issue, making one reason why you should consult a dentist. They can check it out and tell you what to do. Wisdom teeth problems often show up as pain. It’s essential to take care of it early to avoid more issues.
- Difficulty in Opening Mouth
Difficulty in opening your mouth or experiencing pain could indicate issues with your wisdom teeth. This might happen because the teeth are growing funnily or causing swelling. Seeing a family dentist can help you to know how to fix it and how to take care of it. A dentist can discover why your mouth is having an issue to open and help you with the proper treatment.
- Gum Inflammation and Swelling
Ignoring gum problems can lead to more significant mouth issues, so getting help early is good. Your family dentist can look closely and suggest treatments. Taking care of gum issues early on can stop more substantial difficulties and keep your mouth healthy.
- Jaw Stiffness
Feeling stiff or in pain around your jaw, especially near your wisdom teeth, might indicate a problem. A family dentist can check if your wisdom teeth are causing the trouble. Finding out early can prevent the problem from worsening and affecting your jaw.
Getting professional advice for jaw stiffness means finding out what’s wrong and getting help to feel better.
- Difficulty in Cleaning
Because wisdom teeth are in a tricky spot, cleaning them well can be challenging. This raises the chances of getting cavities and gum problems. Talking to your family dentist is a good idea if you struggle to clean your wisdom teeth. They can teach you the best way to clean them and suggest things to help.
Your dentist can show you how to clean your wisdom teeth, stopping future dental problems.
- Tooth Crowding
Wisdom teeth showing up can sometimes crowd your other teeth or move around. If you notice changes in how your teeth fit together, talking to a family dentist is smart. They can check if your wisdom teeth are the reason and suggest things to fix the problem. Getting help early can stop issues with your teeth fitting together and keep them in the right place.
Family dentistry likes to prevent problems, so dentists can monitor how your wisdom teeth grow. Regular check-ups mean finding issues early and getting help if needed.
Conclusion
Whether your teeth hurt or you want to be careful, family dentistry has the answers for dealing with wisdom teeth issues. Schedule regular check-ups, pay attention to how your mouth feels, and talk to your dentist for personalized advice on caring for your wisdom teeth.

by Liam Snow
Decoding Leach Fields: Usage, Maintenance, and Optimization
Leach fields, also known as drain fields, are a critical component of septic systems, playing a crucial role in the disposal and filtration of wastewater. Understanding how leach fields function, their proper usage, and the importance of maintenance is vital for homeowners relying on septic […]
Beauty & FashionLeach fields, also known as drain fields, are a critical component of septic systems, playing a crucial role in the disposal and filtration of wastewater. Understanding how leach fields function, their proper usage, and the importance of maintenance is vital for homeowners relying on septic systems. This article aims to Decoding Leach Fields, offering valuable insights into their usage, maintenance, and optimization for the audience in Canada.

Understanding Leach Fields
1. Purpose and Functionality
- Wastewater Filtration: Leach fields are designed to filter and distribute treated wastewater into the soil.
- Final Stage: After wastewater undergoes treatment in the septic tank, the clarified effluent is released into the leach field for further purification.
2. Location and Design
- Below Ground: Leach fields are typically located underground, covering a significant area.
- Pipe Network: Perforated pipes distribute the treated wastewater evenly across the leach field.
- Soil Interaction: The soil in the leach field absorbs and treats the effluent, providing a natural filtration process.
Proper Usage of Leach Fields
1. Waste Disposal Practices
- Septic-Friendly Products: Use septic-safe household products to avoid harmful chemicals that can disrupt the microbial balance in the septic tank and leach field.
- No Hazardous Waste: Refrain from disposing of hazardous materials in sinks or toilets as they can harm the septic system.
2. Water Conservation
- Moderate Water Usage: Excessive water usage can overload the septic system, affecting the efficiency of the leach field.
- Spread Out Water Usage: Avoid large amounts of water usage in a short period, such as consecutive loads of laundry, to prevent system overload.
Maintenance Practices for Leach Fields
1. Regular Inspections
- Professional Inspections: Schedule regular inspections by septic tank services to assess the overall health of the septic system.
- Visual Checks: Homeowners can observe the leach field area for any signs of pooling water or foul odours.
2. Pumping the Septic Tank
- Scheduled Pumping: Regular septic tank pumping prevents the accumulation of solids that may harm the leach field.
- Professional Services: Hire experienced septic tank services to perform proper pumping and maintenance.
3. Avoiding Heavy Loads
- Limit Heavy Equipment: Avoid driving heavy vehicles or equipment over the leach field, as it can compact the soil and hinder proper water absorption.
- Landscaping Caution: Exercise caution when landscaping around the leach field to prevent damage to pipes and soil.
Optimizing Leach Field Performance
1. Soil Aeration
- Aeration Practices: Implement soil aeration techniques to enhance the absorption capacity of the leach field.
- Professional Consultation: Seek advice from septic tank services on appropriate aeration methods.
2. Planting Grass
- Grass Cover: Planting grass over the leach field helps prevent soil erosion and encourages natural filtration.
- Avoid Trees and Shrubs: Refrain from planting deep-rooted trees or shrubs that may interfere with the leach field infrastructure.
Conclusion
Decoding leach fields is crucial for Care for Your Septic System. Proper usage, regular maintenance, and optimization practices contribute to the longevity and effectiveness of leach fields. Homeowners should stay informed about best practices to ensure the reliable performance of their septic systems.
For homeowners in Canada looking to ensure the optimal performance of their septic systems and leach fields, it is essential to engage with experienced septic tank services. These professionals can provide expert advice, conduct regular inspections, and offer maintenance services to keep the septic system in top condition.
Take the proactive step toward a well-maintained septic system; connect with reputable septic tank services for reliable support and guidance.

How Audio-Visual Solutions Impact Retail Environments
In today’s rapidly evolving retail landscape, creating a memorable and engaging shopping experience has become more crucial than ever. As consumer expectations continue to grow, retailers are seeking innovative ways to capture their attention and foster a strong connection. One such impactful approach is the […]
BusinessIn today’s rapidly evolving retail landscape, creating a memorable and engaging shopping experience has become more crucial than ever. As consumer expectations continue to grow, retailers are seeking innovative ways to capture their attention and foster a strong connection. One such impactful approach is the integration of audio-visual solutions within retail environments. By harnessing the potential of audio-visual technology, retailers can transform their spaces into immersive and dynamic settings that leave a lasting impression on customers.
This article delves into the significance of audio-visual solutions in enhancing the retail experience and explores how they influence customer engagement, brand perception, and overall sales.
Enhancing Engagement Through Immersion
In a world inundated with visual stimuli, creating an immersive environment is paramount for capturing customers’ attention. Audio-visual solutions offer a multifaceted approach to engagement, leveraging both sight and sound to draw customers into the retail experience. By integrating large, high-definition displays and strategically placed speakers, retailers can create captivating visual and auditory displays that tell a compelling brand story.
- Visual Storytelling: Immersive visuals provide an opportunity to convey a brand’s ethos and message seamlessly. High-quality displays can showcase product features, benefits, and real-world applications, allowing customers to envision the role of the product in their lives.
- Sensory Appeal: The synergy of audio and visual elements stimulates multiple senses simultaneously, creating a more profound and memorable experience. Studies have shown that multisensory engagement leads to increased emotional attachment to brands and products.
- Interactive Touchpoints: Touchscreen displays and interactive kiosks enable customers to explore products and information in a hands-on manner. This interactive engagement fosters a sense of involvement and empowerment, helping customers make more informed purchasing decisions.
Influencing Brand Perception
A well-executed audio-visual strategy not only engages customers but also shapes their perception of a brand. By orchestrating a harmonious blend of aesthetics, sound, and messaging, retailers can establish a distinct brand identity that resonates with their target audience.
- Elevating Aesthetics: Strategic placement of displays and lighting can enhance the ambiance of retail space. The atmosphere created through audio-visual solutions influences how customers perceive the brand’s sophistication, modernity, and attention to detail.
- Consistency and Coherence: A consistent audio-visual experience across different touchpoints reinforces the brand’s identity and messaging. Whether it’s an in-store display, online video content, or social media campaign, maintaining a unified presentation reinforces the brand’s credibility and reliability.
- Personalization: Advanced audio-visual solutions can be tailored to individual customer preferences. For instance, digital signage can display personalized recommendations based on a customer’s past purchases or browsing history, demonstrating the brand’s understanding and care for its customers.
Boosting Sales and Loyalty
The integration of audio-visual solutions has a direct impact on a retailer’s bottom line. Engaged and emotionally connected customers are more likely to make purchases and become loyal brand advocates.
- Upselling Opportunities: Immersive displays can showcase complementary products and upsell opportunities in an unobtrusive manner. By suggesting products that align with the customer’s current choices, retailers can increase the average transaction value.
- Dwell Time Extension: Engaging audio-visual content can extend customers’ time in-store, providing more opportunities for them to discover products and make purchases.
- Shareable Experiences: Memorable retail experiences are often shared through word-of-mouth and on social media. When customers are delighted by innovative audio-visual displays, they are more likely to share their experiences, effectively becoming brand ambassadors.
Conclusion
The integration of audio-visual solutions in retail environments represents a transformative shift in how brands engage with their customers. By leveraging immersive visuals and sound, retailers can create memorable experiences that resonate with customers on an emotional level.
Incorporating audio-visual solutions into your retail space has the potential to elevate your brand’s impact and customer engagement. To explore how audio-visual solutions can be tailored to your unique brand identity and customer preferences, contact us for a consultation. Let us help you transform your retail environment into an immersive, dynamic, and unforgettable shopping experience that keeps customers coming back for more.
Remember, the world of retail is changing, and embracing audio-visual solutions could be the key to staying ahead in this dynamic landscape.

by Liam Snow
What kind of injuries are typically claimed in injury suits?
When someone hurts you, you can ask for money to help with your injuries. This request is called an injury suit. Long-term disability lawyers in Brampton often handle these suits. They cover various incidents causing harm. People file these claims to cover injury costs. This […]
healthWhen someone hurts you, you can ask for money to help with your injuries. This request is called an injury suit. Long-term disability lawyers in Brampton often handle these suits. They cover various incidents causing harm. People file these claims to cover injury costs. This is where individuals seek compensation for the damage they’ve suffered. Let’s explore the common types of injuries that lead to these suits in simple Canadian English.
Car Accidents
Car accidents are a common reason for these cases. People can get hurt in crashes, leading to hefty medical bills. Lawyers who focus on injury cases guide individuals through the legal process. They help them obtain the necessary money for recovery.
Slip and Fall Accidents:
People might get hurt if they slip and fall because the place is not safe. In such cases, the injured person may seek compensation. They can request money for injuries and lost wages if they can’t work. Specialized injury lawyers assist and guide them through this claim process.
Medical Malpractice
Doctors or nurses making mistakes can lead to harm. This harm may result in an injury case. Patients might ask for money to cover medical costs and for the pain they went through. Disability lawyers are experts at handling these situations. They work on helping patients step by step to get the right amount of money.
Work-Related Injuries
People might file a case for work-related injuries. If someone gets hurt at work, they can claim money for medical bills and lost wages. Specialized injury lawyers help them build a strong case.
Product Defects
If something a person bought is dangerous and causes harm, they might file a case against the company that made it. This is called a product defect case. Disability lawyers play a significant role in ensuring these companies are responsible and helping the person who got hurt.
Assault
The injured person might file a case when someone intentionally hurts another person. They could ask for money to help with their medical bills and the pain they went through. Lawyers specializing in injury cases guide them through the legal process.
Dog Bites
If a dog hurts someone, the injured person might file a case. They could claim money for their medical bills and lost wages because they couldn’t work. Lawyers specializing in injury cases assist in these cases. They work on helping victims get the proper compensation for their injuries and losses.
Conclusion
Injury suits cover different situations where people get hurt. Lawyers who specialize in injury cases help with other conditions. They assist in cases like car accidents and slips and falls. These lawyers are also experts in dealing with injuries caused by doctors’ mistakes and work-related incidents. You can also contact them if you suffer from the effects of defective products, assault, and dog bites. In Brampton, long-term disability lawyers play a significant role in helping through these challenging situations. They can ensure a smooth legal process and fair compensation.

8 Key Garage Door Maintenance Tips That Can Help Prevent Costly Future Repairs
Every homeowner understands the importance of maintaining their home, but the garage door is often overlooked. However, this essential part of our homes provides safety, security, and convenience. Regular maintenance from garage door experts can help prevent costly future repairs and extend the life of […]
Home ImprovementEvery homeowner understands the importance of maintaining their home, but the garage door is often overlooked. However, this essential part of our homes provides safety, security, and convenience. Regular maintenance from garage door experts can help prevent costly future repairs and extend the life of your garage door. Here are eight key garage door maintenance tips that every homeowner should know.
Regularly Inspect the Garage Door’s Hardware
Look for loose bolts and brackets holding your garage door and tracks together. Over time, these can loosen due to the door’s movements. Tighten them with a socket wrench to ensure they are secure and your door functions well.
Lubricate the Moving Parts
Your garage door has several moving parts that need regular lubrication. Applying a high-quality lubricant to hinges, rollers, and springs will keep them working smoothly and prevent wear and tear. Just a few minutes spent on this task can save you from expensive repairs in the future.
Check the Garage Door Balance
An unbalanced garage door will have to work harder, which can lead to a shorter lifespan. Disconnect the opener and manually lift the door halfway up to check the balance. If it doesn’t stay in place, it means the springs might need adjustment by a professional.
Ensure the Garage Door Opener Works Properly
Your garage door opener plays a key role in operating your door. Check for signs of trouble, such as slow movement, strange noises, or incomplete opening. If you notice any of these, consult a garage door repair service to avoid potential issues.
Clean the Garage Door Regularly
Regular cleaning can prevent rust and corrosion. Use a mild detergent and a soft sponge or cloth to clean the door. Don’t forget to rinse thoroughly. Repainting your door can add an extra layer of protection and keep it looking fresh.
Check the Door’s Weatherstripping
The weatherstripping at the bottom of your garage door helps keep out rain, snow, and cold air. Regularly inspect it for wear and replace if needed. It not only protects your garage contents but also increases energy efficiency.
Inspect and Replace the Rollers
Whether your door has steel or nylon rollers, you should inspect them twice a year. Look for signs of wear, chips, or cracks. If they are damaged, consider replacing them to ensure your door operates smoothly.
Test the Garage Door’s Safety Features
Most garage doors have safety features like auto-reverse and motion sensor functions. Test these regularly to ensure they work correctly. A non-functioning safety feature can be dangerous and lead to accidents.
Maintain Your Door, Secure Your Home
In conclusion, maintaining your garage door isn’t a daunting task, but it is crucial for the safety and longevity of your door. Regularly following these eight simple steps can help prevent major problems and costly repairs in the future. Remember, always call a professional if you ever feel unsure about a garage door issue. After all, a well-functioning garage door keeps your property secure and ensures peace of mind. Be proactive, maintain your garage door, and enjoy its benefits.

Different Types of Garage Doors for Your Home: A Quick Guide
Garage doors play a crucial role in enhancing the aesthetics and security of your home. Choosing a new garage door can be overwhelming, especially with the various types of garage doors available in the market. In this quick guide, we will explore the different types […]
Home ImprovementGarage doors play a crucial role in enhancing the aesthetics and security of your home. Choosing a new garage door can be overwhelming, especially with the various types of garage doors available in the market.
In this quick guide, we will explore the different types of garage doors to help you make an informed decision.

Types of Garage Doors
There are four main types of garage doors. These include:
- Sectional Garage Doors: These are the most popular garage doors in Canada. They are made of horizontal panels that slide upwards and rest parallel to the ceiling when open. Sectional garage doors are available in various materials, including steel, aluminum, and wood.
- Roller Doors: Roller doors are made of a single curtain of material that rolls up and down around a drum. They are suitable for homes with limited headroom, as they do not require overhead space.
- Side-Hinged Garage Doors: These doors open outwards like traditional doors and are ideal for garages with limited headroom. Side-hinged garage doors are available in various materials, including steel, wood, and aluminum.
- Up and Over Garage Doors: Up and over garage doors swing outwards and then upwards to open. They are available in various materials and are suitable for homes with ample headroom.
Material Options
The material of your garage door is an important consideration when choosing a new garage door. The following are the common materials used to make garage doors:
- Steel: Steel garage doors are durable, low maintenance, and affordable. They are available in various styles, colours, and finishes, making them a popular option for homeowners.
- Wood: Wood garage doors provide a natural and warm look to your home. They are available in various styles and finishes, including cedar, hemlock, and mahogany. However, they require more maintenance than other materials and are more expensive.
- Aluminum: Aluminum garage doors are lightweight, low maintenance, and durable. They are available in various styles and finishes, including wood grain finishes. However, they are less energy-efficient than other materials.
- Fibreglass: Fiberglass garage doors are lightweight, durable, and low maintenance. They are available in various finishes, including wood grain finishes. However, they are less durable than other materials and can crack or fade over time.
Additional Features
When choosing a new garage door, you may also want to consider the following additional features:
- Insulation: Insulated garage doors can help to improve energy efficiency, reduce noise, and provide additional strength to the door. Insulation is especially important if you use your garage as a living space or have rooms above the garage.
- Windows: Garage doors with windows can help to improve the aesthetics of your home and provide natural light to your garage. However, they may compromise your privacy and security.
- Security: Garage doors with additional security features, such as tamper-resistant locks and alarms, can help to enhance the security of your home.
Conclusion
Choosing a new garage door can be overwhelming, but with this quick guide, you can make an informed decision. Consider the type of garage door, material options, and additional features before making your final decision. A new garage door can enhance the aesthetics and security of your home, so choose wisely.
If you are looking for a professional and reliable garage door installation service in Canada, contact us today. Our team of experts can help you choose the right garage door for your home and provide professional installation services. Choose us for quality service and guaranteed customer satisfaction.

How to Give the Right Flowers as Thank You Gift
Looking for a unique way to say “thank you” to someone special? Well, have you ever considered the magic of flowers? Flowers can convey heartfelt gratitude like no other gift. You can even send a thoughtful thank-you gift by arranging a birthday flower delivery! But […]
flowersLooking for a unique way to say “thank you” to someone special? Well, have you ever considered the magic of flowers? Flowers can convey heartfelt gratitude like no other gift. You can even send a thoughtful thank-you gift by arranging a birthday flower delivery! But how do you choose the right flowers to express your appreciation? Here are idea to choose the right flowers as thank you gift
Consider the Recipient’s Preferences
When selecting flowers as a thank-you gift, it’s important to consider the person you are giving them to. What colours do they like? Do they have a favourite type of flower? By considering their preferences, you can make your gift even more meaningful. You can always choose classic and universally loved flowers like roses or daisies if you still decide.
Express Emotions with Flower Meanings
Did you know that flowers can have different meanings? Each type of flower can convey a specific emotion or sentiment. For example, roses symbolize love and gratitude, while sunflowers represent happiness and warmth. Learn about flower meanings and language flowers and choose the ones that align with your thank you message. It will make your gift extra special.
Think about the Occasion
Consider the occasion when giving flowers as a thank-you gift. Is it a birthday, a wedding, or a simple gesture of appreciation? The occasion can guide your flower choice. For instance, if it’s a birthday, you can add a festive touch by selecting bright and vibrant flowers. On the other hand, elegant flowers like orchids or lilies might be a better choice for a more formal occasion.
Personalize with a Handwritten Note
Remember to include a heartfelt handwritten note along with the flowers. A simple “thank you” can go a long way in expressing your gratitude. Take a moment to write a few kind words about why you are grateful and how much the person means to you. It will make your gift even more meaningful and show that you put thought and effort into it.
Final Words
Choosing the right flowers as a thank-you gift is a thoughtful gesture that can make someone feel appreciated. By considering the recipient’s preferences, flower meanings, and the occasion, you can create a personalised gift that will leave a lasting impression. Remember to accompany your flowers with a handwritten note to express your gratitude in words.
Giving flowers as a thank-you gift is a beautiful way to show appreciation, whether for a friend, a family member, or a teacher. So, next time you want to say “thank you,” consider the power of flowers. They will surely bring a smile to the recipient’s face and warm their heart.
Why not brighten someone’s day today? Take a moment to think of someone who has done something kind for you and deserves a thank-you gift. Choose a beautiful bouquet, write a heartfelt note, and make your day memorable. The act of gratitude brings joy to others and fills our hearts with happiness. So go ahead, and spread some cheer with the perfect thank-you gift of flowers!

by Liam Snow
17 Reasons Why Inbound Marketing Enables Local Businesses To Dominate Their Competitors
Inbound marketing is a great way for local businesses to get more customers, gain trust, and stand out from their competitors. Here are 17 reasons why inbound marketing is beneficial for local companies: 1. Improves visibility Inbound marketing helps your business get noticed by creating […]
BusinessInbound marketing is a great way for local businesses to get more customers, gain trust, and stand out from their competitors. Here are 17 reasons why inbound marketing is beneficial for local companies:

1. Improves visibility
Inbound marketing helps your business get noticed by creating fun and informative content. When you reach more people, it helps more people learn about your brand and makes your company more visible online.
2. Provides valuable information
Content marketing educates prospects about their interests. It helps build trust and keeps customers coming back to your brand.
3. Generates more leads
Inbound attracts visitors who convert into leads through calls-to-action. Improve online visibility through natural search engine optimization in Toronto.
4. Converts lead into customers.
Nurtured leads convert at higher rates. Inbound’s multi-touch process builds relationships with prospects.
5. Retains existing customers
Current customers who engage with your brand through inbound are more likely to stay loyal.
6. Lower cost than outbound
Inbound focuses on organic marketing activities, which cost less than disruptive outbound tactics.
7. Improves SEO rankings
Quality content and inbound links signal search engines to rank your pages higher.
8. Builds authority and trust
Inbound establishes your company as an industry authority. This trust makes customers more likely to buy from you.
9. Creates brand advocates
Satisfied inbound customers happily refer others if you provide a good experience. Referrals are powerful.
10. Adapts to mobile users
Inbound is designed for mobile, the primary way people consume content today. It is key for local reach.
11. Aligned with buyer journey
Inbound guides visitors through awareness, consideration, and decision—how people buy now.
12. Flexibility across channels
Inbound efforts across online and offline platforms boost brand visibility. You may reach more people and boost your online and offline exposure by using many channels.
13. Ongoing process, not one-off
For long-term success, consistent inbound actions that compound results over time are essential.
14. Levels playing field
Inbound gives small businesses the same digital access as large companies to attract customers.
15. Adapts to changing technology
Inbound evolves as new marketing channels emerge. This built-in flexibility keeps you competitive.
16. Measurable results
Inbound efforts across online and offline platforms boost brand visibility. You may reach more people and boost your online and offline exposure by using many channels.
17. Customizable strategies
With numerous inbound tactics, you can develop a unique approach tailored to your business.
Conclusion:
Inbound marketing is a proven way for local businesses like yours to attract more customers without spending too much money. It helps you share important information with the right people at the right time by creating useful content on different platforms.
Choose a few inbound strategies that fit your business and resources. Start with a company blog, SEO optimization, email marketing, and social media engagement. Track your efforts and double down on what works. Commit to inbound marketing and watch your visibility, leads, and sales start to climb. When inbound is done right, it’s a game-changer for dominating local competitors. You got this!

by Randal Spain
Navigating the Landscape of Online Couple Therapy
In today’s fast-paced world, relationships are often put to the test by various challenges that arise. Whether it’s communication issues, conflicts, or the struggle to balance personal and professional lives, many couples find themselves seeking guidance to navigate these obstacles and strengthen their bonds. This […]
healthIn today’s fast-paced world, relationships are often put to the test by various challenges that arise. Whether it’s communication issues, conflicts, or the struggle to balance personal and professional lives, many couples find themselves seeking guidance to navigate these obstacles and strengthen their bonds. This is where online counselling for couples comes into play, offering a convenient and effective way for partners to work on their relationships in the digital age. This article explores the landscape of online couple therapy, its benefits, considerations, and how it can provide a path towards healthier and more fulfilling relationships.
The Rise of Online Counselling for Couples
Traditional face-to-face therapy has long been a cornerstone for relationship help, but with the advent of technology, online counselling for couples has gained significant traction. This approach offers several advantages that make it an attractive option for many Canadians:
- Convenience: The digital nature of online couple therapy eliminates the need for couples to travel to a therapist’s office. This is particularly beneficial for those residing in remote areas or having busy schedules.
- Flexibility: Online sessions can be scheduled to accommodate the couple’s commitments, whether it’s work, childcare, or other responsibilities. This flexibility can contribute to a more relaxed and focused atmosphere during therapy.
- Access to Specialized Professionals: Couples facing unique challenges, such as long-distance relationships, can benefit from accessing therapists with specific expertise in these areas, which might not be locally available.
- Reduced Stigma: For some, seeking therapy might carry a certain stigma. Online counselling allows couples to engage in therapy discreetly from the comfort of their own homes.
Benefits of Online Counselling for Couples
Online couple therapy offers a range of benefits that contribute to its growing popularity among Canadians seeking relationship assistance:
- Enhanced Comfort: Couples often find it easier to open up about sensitive topics in familiar surroundings, which can lead to more productive therapy sessions.
- Communication Improvement: A common challenge in relationships is communication breakdown. Online therapy provides a platform for partners to practice healthy communication techniques with the guidance of a professional.
- Time and Cost-Efficiency: With no need to commute, online therapy saves time and money. This can encourage couples to commit to regular sessions, leading to more sustainable progress.
- Record Keeping: Online sessions can be recorded with consent, allowing couples to revisit discussions and insights between sessions.
- Couples’ Participation: Engaging in therapy from home can encourage equal participation from both partners, fostering a sense of shared responsibility for the relationship’s growth.
Considerations for Online Couple Therapy
While online counselling for couples offers numerous advantages, there are factors to consider to ensure a successful experience:
- Technology and Privacy: Stable internet connection and private space are essential for uninterrupted sessions. Couples should also prioritize platforms that prioritize confidentiality and data security.
- Choosing the Right Therapist: Just like in traditional therapy, finding the right therapist is crucial. Couples should research therapists’ qualifications, reviews, and expertise to ensure compatibility.
- Self-Discipline: Engaging in online therapy demands self-discipline. Couples should commit to attending sessions and completing recommended exercises outside of the sessions.
Conclusion
Navigating the landscape of counselling for couples provides a pathway to healthier, more fulfilling relationships. The convenience, flexibility, and personalized guidance online offers align well with the needs and lifestyles of modern Canadians. As technology continues to reshape our lives, embracing online couple therapy can be a pivotal step toward overcoming relationship challenges.
If you and your partner are seeking to enhance your relationship and overcome hurdles, consider exploring the realm of online counselling for couples. With its numerous benefits and the opportunity to work with experienced professionals, online therapy can provide the support and tools you need to build a stronger, more harmonious bond.

6 Key Drawbacks of Mobile Phone Garage Door Apps
Garage door apps are becoming more popular because of their convenience. With just a few taps, you can open or close your garage door from anywhere. But, like all technologies, they have their downsides too. Here are six key drawbacks of mobile phone garage door […]
Home ImprovementGarage door apps are becoming more popular because of their convenience. With just a few taps, you can open or close your garage door from anywhere. But, like all technologies, they have their downsides too. Here are six key drawbacks of mobile phone garage door apps.
These Apps Depend on Your Phone’s Battery Life
One of the main disadvantages of mobile phone garage door apps is that they depend on your phone’s battery life. If your phone’s battery dies, you could be locked out of your garage. In this situation, you need reliable garage door repair & services.
It could be particularly problematic if you return home late at night or during adverse weather conditions. So, while the app adds convenience, you must be extra cautious about keeping your phone charged, especially when you’re out and about.
Garage Door Apps Need a Reliable Internet Connection
Mobile phone apps rely on a stable internet connection to work. If your home’s Wi-Fi network is down or you’re trying to operate the door remotely but don’t have a good signal, you might be unable to open or close your garage door.
This dependence on internet connectivity can be inconvenient and frustrating. It can be hard in emergencies when you need to access your garage immediately.
There Is a Risk of Phone Loss or Theft
While it’s unlikely, there’s always the risk that you could lose your phone or have it stolen. If this happens, someone else could access your garage door app and, by extension, your home. While there are ways to protect your phone and app, such as using strong passwords and biometric security features, it’s still a risk to consider.
There Is a Possibility of App Malfunctions
Garage door apps can experience bugs or glitches like any software. The app could freeze, crash, or stop working for no clear reason. These technical issues can be frustrating, especially when getting into your garage quickly. Even though developers often fix these issues with updates, there’s still the chance of encountering problems.
Some Apps May Not Be User-Friendly
Not all mobile phone apps are created equal. Some are intuitive and easy to use, while others may be more complex. If the app is difficult to navigate or has a steep learning curve, it could make accessing your garage more complicated rather than easier. You might struggle with the app when you’re in a hurry, which could lead to frustration and stress.
There May Be Compatibility Issues with Garage Door Apps
Finally, there may be compatibility issues between your phone and the garage door app. Some apps may not work well with certain operating systems or phone models. If you upgrade your phone or switch to a different operating system, you might need to find a new app or change your garage door system. It could be costly and inconvenient.
Choose the Right Garage Door Carefully
While such garage doors offer benefits, it’s essential to consider these drawbacks. Evaluate your specific situation, your comfort level with technology, and the reliability of your phone and internet connection. If you feel these drawbacks outweigh the benefits. Sticking with a traditional remote or seeking other garage door-opening solutions might be best. Remember, it’s all about finding a solution that fits your lifestyle and needs. Consider consulting with a garage door professional to explore your options and make the best decision for your home.

The Lastest on Hormone Replacement Therapy Safety
First, what are hormones? Hormones are chemical substances produced in the endocrine glands and released into the bloodstream to control the activity of cells and organs. The two key hormones in the female body are progesterone and estrogen. Imbalance estrogen levels have been linked to […]
healthFirst, what are hormones? Hormones are chemical substances produced in the endocrine glands and released into the bloodstream to control the activity of cells and organs. The two key hormones in the female body are progesterone and estrogen. Imbalance estrogen levels have been linked to many health conditions, including endometriosis, fibroids, infertility, fibrocystic breast, cancer, and premenstrual syndrome (PMS). In this blog we will learn about hormone replacement therapy safety. So, First what are hormones read more here to know
female hormone that induces the growth of tissues
Although estrogen is mainly produced in the ovaries, it is also made in other parts of the body, like the liver, adipose cells, breast tissue, and adrenal glands. Estrogen is the female hormone that induces the growth of tissues, and in doing so, it impacts bone formation, libido, breast tissue, and the lining of the womb and vaginal walls
Estrogen three different forms
Estrogen occurs in three different forms in the human body: (i) Estrone (E1), (ii) Estradiol (E2), and (iii) Estriol (E3). Estrone is the predominant form of estrogen during menopause. It is responsible for sexual development and function in women. Estradiol (E2) is the most powerful form of the estrogen hormone. It is produced in large amounts throughout a woman’s reproductive life. Estriol is the least powerful form and reaches its peak during pregnancy. It’s very important that estrone and estradiol are metabolized in a healthy way to prevent their side effects. The metabolism of the estrogen hormone is dependent on the liver and adrenal function.
Check hormone levels begain therapy
Before you begin therapy to adjust or supplement hormone levels, it is crucial to undergo tests to know the baseline levels of the hormones and their metabolites in circulation. Hormone blood tests are not the most precise at evaluating the baseline levels of these hormones. More accurate results can be obtained through tests that check the metabolites of each hormone in urine and saliva. You may speak to your naturopathic doctor to know if this kind of testing suits your particular health conditions.
Check health conditions before therapy
There are some health conditions where it is necessary to supplement with hormones to manage symptoms and improve the quality of life. Bio-identical hormones are known to be safer than synthetic hormones, such as Premarin and Provera. The reason is that, unlike synthetic hormones, bio-identical hormones have the same molecular structures as those produced by the body and act the same way as endogenous hormones at the receptor site. Bio-identical hormones are quickly broken down after use and are recommended in physiological quantities.
conclusion
According to studies and some professionals in canada, bioidentical hormones protect breast tissue and lower the risk of breast cancer than synthetic hormones (those synthesized from animal sources). Research has also shown that bioidentical hormones protect against cardiovascular diseases.
Women are likely to suffer from hormone-related conditions all through their reproductive life. Running away from hormone therapy is not the answer to managing the symptoms you may be experiencing. The best way to handle symptoms and maintain a good quality of life is to make the right decision about what’s best for your health.

Understanding the Meaning Behind Different Types of Flowers
Flowers have been used to show feelings and send messages for a long time. Flowers have a way of showing feelings that can’t be said with words alone. It is true for both romantic and sad gestures. You must know each flower’s meaning to choose […]
Home ImprovementFlowers have been used to show feelings and send messages for a long time. Flowers have a way of showing feelings that can’t be said with words alone. It is true for both romantic and sad gestures. You must know each flower’s meaning to choose the right gift for any event. Knowing these meanings can help you get the right flowers for each occasion. It also makes ordering fresh flowers online easy because you know what you want. We will discuss about meaning behind different types of flowers.

Rose
The rose is undoubtedly one of the best-known and most popular flowers. Red roses are often associated with love and longing. They are often chosen for romantic events like Valentine’s Day or anniversaries. On the other hand, white roses are often given as gifts at weddings or as a sign of sympathy. They are thought to represent purity and innocence. Yellow roses, conversely, are thought to mean jealousy and cheating. Pink roses mean grace, happiness, and kindness.
Tulips
Tulips are another popular flower that comes in many different forms. Each of which means something different. Yellow tulips are thought to represent happiness. They are often given as a sign of friendship, while red tulips are thought to represent love. Purple is often linked with royalty. It makes it a good choice for more serious events and celebrations. White tulips are a sign of forgiveness. You can give them to ask for forgiveness or to show that you’re willing to forgive.
Lily
The lily is a stunningly beautiful and elegant flower that stands for purity and quiet beauty. Lilies come in many different colours. White lilies are a sign of modesty and purity. Orange lilies are a sign of love, and yellow lilies signify happiness. People often think of the lily of the valley as a sign of gentleness and purity of the heart.
Carnations
Carnations are another type of flower in different colours, and each colour has its meaning. Pink carnations are a sign of thanks and are often given to show respect. Red is linked to love and respect, while white is linked to purity and luck.
Daisy
The daisy is a flower that stands for innocence and purity because it is simple and sweet. They are often given to new moms to show thanks or as a gift when a baby is born.
Sunflowers
Sunflowers, known for being happy and looking bright, have come to stand for love and loyalty. They are often given as a gift to a friend or family member to say thanks.
Certain kinds of flowers are better for certain events. For example, red roses are a classic choice for Valentine’s Day. Pink tulips and begonias are great ways to show appreciation on Mother’s Day or Father’s Day. You can give these flowers on either holiday. Sunflowers are a great way to cheer someone up on a bad day. White lilies are often used at weddings and when someone wants to show sadness.
Final words on the Meaning Behind Different Types of Flowers
Flowers are an elegant gift that can be given for any event because they are beautiful and thoughtful. If you choose the right flower, you can send the desired message and show the feeling you want. Knowing the different kinds of flowers mean you can choose the best flowers for any occasion. Whether to celebrate a big accomplishment or grief, look for flowers that suit each occasion.

by Liam Snow
How to Manage Your Stress and Anxiety If You’re Accused of a Crime
Being accused of a crime can be overwhelming. Stress and anxiety are natural reactions in such situations. But, managing these emotions is vital to dealing with the situation. Here, we explore practical steps to cope with stress and anxiety and navigate this challenging time with […]
Law & LegalBeing accused of a crime can be overwhelming. Stress and anxiety are natural reactions in such situations. But, managing these emotions is vital to dealing with the situation. Here, we explore practical steps to cope with stress and anxiety and navigate this challenging time with composure.

Identify Your Emotions to Address the Issue
Recognize and accept your feelings. It’s normal to feel scared, angry, or confused. Admitting these emotions is the first step towards managing them. Acknowledge the stress and anxiety. Remember, it’s okay to have these feelings.
Talk with Someone to Lighten the Burden
Talking to someone helps a lot. Speaking about your feelings enables you to understand them better. You’re not alone; talking about it is a brave and helpful step to feel better. Every word you share is a step towards feeling stronger and more transparent.
Learn About the Legal Process to Reduce Uncertainty
Learning about legal steps is important. Knowledge is like a shield that makes you brave and ready. There are helpful resources like books, websites, and people who know much about law. They are ready to help explain complicated things in more straightforward ways. Every fact you learn is like adding a helpful tool to a toolbox. You’re not walking this path alone – help is always there when needed.
Engage a Lawyer to Get Professional Guidance
Look for the affordable solicitors in London. They provide legal advice and support. A lawyer navigates the legal complexities, fights for your rights, and works towards the best possible outcome. They are your ally in this journey.
Focus on Physical Health to Boost Mental Wellbeing
Take care of your physical health. Eat balanced meals, exercise, and get adequate sleep. A healthy body contributes to a resilient mind. Physical wellness is integral to handling stress and anxiety.
Practice Relaxation Techniques to Enhance Emotional Control
Embrace relaxation techniques. Meditation, deep breathing, or progressive muscle relaxation can help. These practices calm the mind and body, clarify, and enhance emotional control. They are tools to combat anxiety.
Avoid Isolation to Encourage Emotional Support
Connect with others. Don’t isolate yourself. Join support groups or communities. Hearing others’ stories and sharing your own fosters support and understanding. It alleviates the feeling of being alone in the ordeal.
Create a Support Network to Strengthen Coping Mechanisms
Build a support network. Surround yourself with people who encourage and uplift you. Their support bolsters your strength to face the challenges ahead. A network provides both emotional and practical help.
Conclusion
Being accused of something serious can make you feel a lot of stress. It’s okay to feel scared or worried. But remember, you can manage these feelings. Recognize what you’re feeling and seek support. Understand what’s happening legally and take care of your health.
Start this positive journey today. Remember, you have control, and people are ready to support you. Every effort you make is a step towards a stronger you. If things seem too much, professional help is available. They can guide and support you through this time. Facing this challenge with calmness and focus is your first victory. Equip yourself with knowledge and support.

The Importance of Regular Dental Checkups for Your Family
Maintaining good oral hygiene is essential for a healthy lifestyle. Regular dental checkups are a crucial aspect of oral care that most people tend to overlook. Dental visits are not just for treating dental problems but also for preventing them from occurring in the first […]
Law & LegalMaintaining good oral hygiene is essential for a healthy lifestyle. Regular dental checkups are a crucial aspect of oral care that most people tend to overlook. Dental visits are not just for treating dental problems but also for preventing them from occurring in the first place. It is highly recommended that everyone in the family, from young children to adults, should have regular dental checkups to ensure good oral health.
In this article, we will discuss the importance of regular dental checkups and why they are necessary for the whole family.
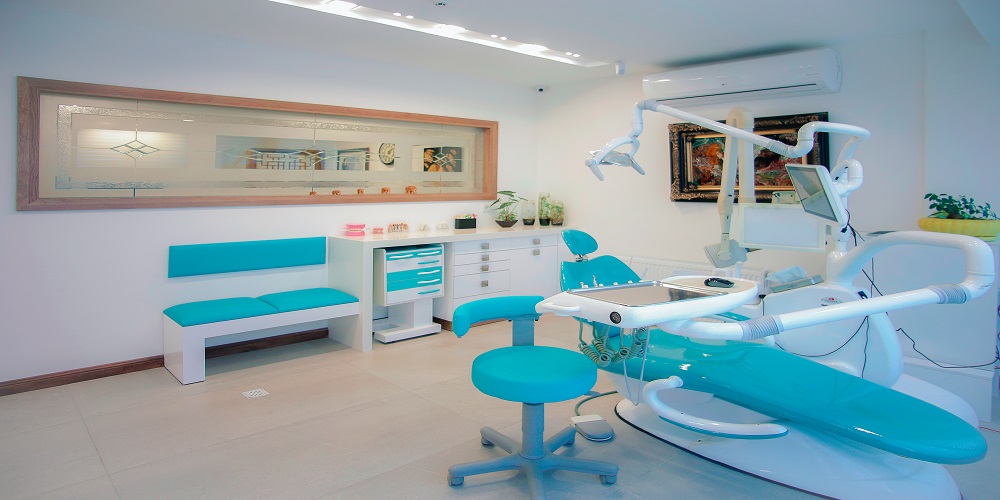
Why are regular dental checkups necessary?
- Early detection of dental problems:
Regular dental checkup help in detecting dental problems at an early stage. The dentist can identify any signs of tooth decay, gum disease, or other oral health issues before they become severe. Early detection of dental problems can help prevent serious complications in the future and save you from unnecessary pain, discomfort, and expenses.
- Prevention of dental problems:
Prevention is always better than cure, and this is especially true in dentistry. Regular dental checkups can help prevent dental problems from occurring in the first place. During the checkup, the dentist will examine your teeth and gums, remove plaque buildup, and provide advice on how to maintain good oral hygiene. The dentist may also recommend fluoride treatment or dental sealants to help prevent tooth decay.
- Personalized oral care:
Every individual has different oral care needs, and regular dental checkups provide personalized oral care tailored to your specific needs. The dentist will examine your teeth and gums and provide advice on how to maintain good oral hygiene based on your oral health condition. Personalized oral care helps in preventing dental problems and maintaining good oral health.
- Improve overall health:
Good oral health is linked to overall health. Poor oral health has been linked to several health problems such as diabetes, heart disease, and stroke. Regular dental checkups can help identify oral health issues that may be affecting your overall health. By maintaining good oral health, you are also contributing to your overall well-being.
Conclusion:
Regular dental checkups are essential for maintaining good oral health. Early detection and prevention of dental problems can save you from unnecessary pain, discomfort, and expenses. Good oral health is linked to overall health, and regular dental checkups can help identify oral health issues that may be affecting your overall health. Therefore, it is highly recommended that everyone in the family, from young children to adults, should have regular dental checkups to ensure good oral health. We provide personalized oral care to ensure good oral health for your family. Our team of experienced and friendly dentists will examine your teeth and gums, identify any oral health issues, and provide advice on how to maintain good oral hygiene. Schedule your appointment today to maintain good oral health for your family.

by Liam Snow
A Comprehensive Guide to Mastering Home Insulation
Home insulation is a crucial aspect of creating a comfortable and energy-efficient living space, especially in a country like Canada, where extreme weather conditions can take a toll on your comfort and energy bills. Whether you’re looking to reduce your heating and cooling costs, increase […]
Home ImprovementHome insulation is a crucial aspect of creating a comfortable and energy-efficient living space, especially in a country like Canada, where extreme weather conditions can take a toll on your comfort and energy bills.
Whether you’re looking to reduce your heating and cooling costs, increase the resale value of your home, or simply create a more comfortable environment, understanding the various insulation techniques and materials is essential.
In this comprehensive guide, we will explore different insulation methods, materials, and tips for improving your home’s insulation. By the end of this article, you’ll be better equipped to make informed decisions about your insulation needs.
The Importance of Home Insulation
Proper home insulation offers a range of benefits:
Energy Efficiency: Insulation helps maintain a stable temperature within your home, reducing the need for excessive heating or cooling, which translates to lower energy bills.
Comfort: Well-insulated homes are more comfortable, with fewer drafts and consistent indoor temperatures.
Environmental Impact: Reduced energy consumption not only saves you money but also contributes to a lower carbon footprint.
Common Insulation Materials
Understanding the different insulation materials available is the first step in mastering home insulation. Here are some commonly used options:
1. Fiberglass Insulation
Description:
Composed of fine glass fibers, fiberglass insulation is widely used for its cost-effectiveness.
Benefits:
Good thermal performance.
Fire-resistant.
Easy to install.
Considerations:
Prone to moisture damage.
Proper installation is crucial.
2. Cellulose Insulation
Description:
Made from recycled paper products, cellulose insulation is an eco-friendly choice.
Benefits:
Excellent thermal performance.
Fire retardant.
Environmentally friendly.
Considerations:
Susceptible to mould and pests without proper treatment.
3. Spray Foam Insulation
Description:
Spray foam insulation is a versatile material that expands to fill gaps and cavities.
Benefits:
Superior insulation properties.
Air and water-tight seal.
Excellent for hard-to-reach areas.
Considerations:
Requires professional installation.
Costlier than other options.
Insulation Techniques
Choosing the right insulation technique is just as important as selecting the appropriate material. Here are some popular methods:
1. Batt Insulation
Description:
Batt insulation comes in pre-cut panels or rolls, making it easy to install between wall studs and ceiling joists.
Benefits:
Suitable for DIY installation.
Cost-effective.
Considerations:
Gaps and improper installation can reduce effectiveness.
2. Blown-in Insulation
Description:
Loose insulation material is blown into wall cavities or attics using specialized equipment.
Benefits:
Ideal for retrofitting existing structures.
Provides excellent coverage.
Considerations:
Requires professional equipment.
3. Spray Foam Insulation
Description:
As mentioned earlier, spray foam insulation is a versatile material that expands to fill gaps and cavities.
Benefits:
Provides an airtight seal.
Effective in hard-to-reach spaces.
Considerations:
Professional installation is necessary.
Tips for Mastering Home Insulation
Now that you have an understanding of insulation materials and techniques, here are some essential tips to keep in mind:
- Consult a Professional: For best results, consult with spray foam insulation specialists or other insulation professionals who can assess your specific needs and provide expert guidance.
- Seal All Gaps: Even the most effective insulation material won’t perform well if there are gaps in your home’s structure. Ensure that all gaps, cracks, and leaks are properly sealed.
- Consider Local Climate: Canada’s diverse climate means that insulation requirements may vary depending on your location. Research local building codes and climate conditions to choose the most suitable insulation.
- Maintain Proper Ventilation: Proper ventilation is essential to prevent moisture buildup and maintain indoor air quality. A well-ventilated home can prevent mould and mildew growth.
Conclusion
Mastering home insulation is a vital step towards creating a comfortable, energy-efficient, and eco-friendly living space in Canada.
If you’re looking to improve your home’s insulation in Canada, contact our trusted insulation experts today for a consultation and professional installation. Create a cozy and energy-efficient home that you’ll love for years to come.
Don’t wait; take the first step towards a better-insulated home now.

The Top 8 Benefits of Regular Adjustments
Chiropractic adjustments, are a form of treatment used by chiropractors. More so to help improve the alignment and function of the musculoskeletal system. Many people may think of chiropractic care as only helpful for those with back pain. But, regular adjustments can provide a wide […]
healthChiropractic adjustments, are a form of treatment used by chiropractors. More so to help improve the alignment and function of the musculoskeletal system. Many people may think of chiropractic care as only helpful for those with back pain. But, regular adjustments can provide a wide range of benefits for people of all people. Increasing the time spent standing throughout the day improves posture and reduce discomfort. This is why to stand more can be helpful in making adjustments. This article will explore the top eight benefits of regular adjustments.
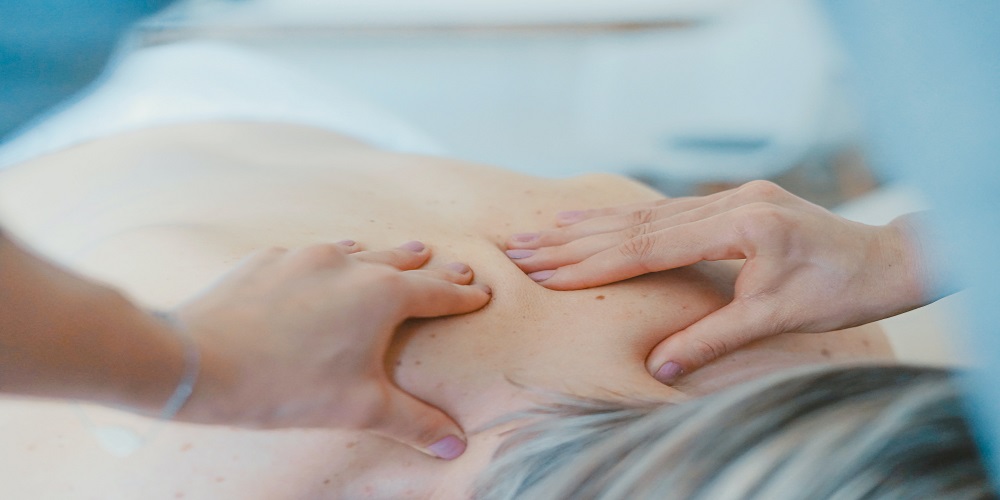
1. Reduced pain and discomfort
One of the most significant benefits of regular adjustments is reducing pain. Chiropractors use gentle techniques to adjust the spine and other joints. This can help to relieve tension, improve circulation, and reduce inflammation. This can be particularly helpful for those who suffer from chronic pain. Also, conditions such as arthritis, fibromyalgia, or migraines.
2. Improved range of motion
Another benefit of regular adjustments is they can help to improve the range of motion in the joints. When joints misalign, it can lead to stiffness and restricted movement. By restoring proper alignment and function, chiropractic adjustments can help to improve flexibility. Range of motion, making it easier to perform daily tasks and take part in physical activities.
3. Better posture
Poor posture is a common problem for many people. Particularly those who spend much time sitting or standing in one position. Regular adjustments can help correct postural imbalances. Improving spinal alignment and reducing the risk of developing chronic pain conditions.
4. Improved immune function
Research has shown that regular adjustments can help to improve immune function. By reducing stress on the nervous system. The nervous system is crucial in regulating the body’s immune response. When functioning, the immune system can better fight off infections and other illnesses.
5. Reduced stress and anxiety
Chiropractic adjustments can also help reduce stress and anxiety. By promoting relaxation and reducing muscle tension. This can be helpful for those who experience high levels of stress in their daily lives. Also, those who suffer from conditions such as anxiety or depression.
6. Improved sleep quality
Many people who receive regular chiropractic adjustments report improved sleep quality. Sleep can impact health and well-being. By reducing tension and promoting relaxation, chiropractic care can help improve sleep quality. Making it easier to fall asleep and stay asleep throughout the night.
7. Increased energy levels
Regular adjustments can also help increase energy levels. By improving spinal function and reducing muscle tension. When the body functions, it requires less energy to perform daily tasks. Leaving more energy for other activities such as exercise or leisure.
8. Better health and wellness
Regular adjustments can help to improve health and wellness of a person. By promoting proper alignment and function of the musculoskeletal system. This can help to prevent injuries, reduce the risk of developing chronic pain conditions. Also, improve quality of life.
Regular adjustments can benefit people of all ages and health backgrounds. If you’re interested in exploring the benefits of chiropractic care for yourself, be sure to speak with a qualified chiropractor in your area. Regular care and maintenance allow you to enjoy improved health. Reduced pain and discomfort, and better overall quality of life.

5 Ways To Weather-Seal A Garage Door
Most garage doors come with weather seals, but with time the rubber or vinyl deteriorates and loosens the garage door. The garage floor may also suffer some damage and could result in the door no longer fitting in its frame. This can lead to substantial […]
Home ImprovementMost garage doors come with weather seals, but with time the rubber or vinyl deteriorates and loosens the garage door.
The garage floor may also suffer some damage and could result in the door no longer fitting in its frame. This can lead to substantial problems as an ill-fitting garage door lets dust, dirt, water, pests, insects, rodents, and several pollutants inside your house.
An effective way to fix the issue is weatherproofing the garage door, so here are 5 different ways you can do it.
5 Ways To Weather-Seal A Garage Door
1. Bottom Seal
This type of rubber seal is fixed right at the bottom of the garage door that molds itself to the floor of the garage, thus sealing it in place. However, it may wear out over time and will have to be replaced.
If the seal is fixed in place with screws, those are to be removed and fitted again to fix the new strip. In some cases the door has a channel at the bottom in which the T-strip or U-strip gasket slides in.
2. Threshold Seal
Threshold seals are attached to the garage floor right where the garage door sits. A strip of rubber or vinyl is measured to fit the garage door from end to end and this strip is then installed on the floor with glue and screws.
Bottom seals and threshold seals can also be used together, preventing water, dust, and dirt from entering the garage.
3. Door Stop Weatherstripping
Depending on the fitting issue, your garage door might need sealing on its sides. For this, the door jamb that is attached to the door frame on the walls on both sides is affixed with rubber or vinyl seal.
The attachment process is similar to a threshold seal, as a weatherstrip is fixed with screws on the jamb while the flap rests on the face of the door.
4. Top Seal
This is a tricky area to work on due to the way the garage door retreats into the top of the door frame when opened. But with the help of some double sided tape, a rubber or brush type seal is installed at the top between the door and the frame.
5. Panel Weatherstripping
V-shaped panel weatherstripping or V-seals are used when there are cracks in the panels of the garage door. The cracks can prove to be detrimental over time if they aren’t covered and sealed properly.
Firstly the length of the cracks is measured and then V-seals are cut according to the same measurement. The best part is that V-seals are self adhesive, and can be simply pasted on the cracks.
Conclusion
All kinds of insects, rodents, and other unwanted elements can easily find their way in from the gaps caused by faulty seals. Therefore it becomes important to ensure that the garage door is properly weather-sealed.
You can opt for any of the listed seals or a combination of seals for this purpose to ensure complete safety at all times. So, contact a garage door expert for weatherproofing without delay!

Is Your Marriage In Trouble: When To Seek Professional Help
Marriage is one of the most significant relationships we experience in life. It can be a source of happiness, companionship, and love. However, sometimes, things do not go as planned, and challenges arise. When issues persist, it is essential to recognize when to seek professional […]
Law & LegalMarriage is one of the most significant relationships we experience in life. It can be a source of happiness, companionship, and love. However, sometimes, things do not go as planned, and challenges arise. When issues persist, it is essential to recognize when to seek professional help to save your marriage.
Recognize The Signs
Couples often face ups and downs in their relationship, but some signs suggest that your marriage may be in trouble.
These signs include:
- Communication Breakdown:
Poor communication, constant arguments, and misunderstandings are common signs of a troubled marriage.
- Emotional Distance:
When one or both partners feel emotionally distant, there is a lack of intimacy, and it can lead to an unhealthy relationship.
- Infidelity:
Infidelity or the suspicion of it can cause trust issues, and if not addressed, can lead to the end of the relationship.
- Growing Apart:
As individuals grow and evolve, it is easy to lose touch with each other’s interests and goals, leading to distance in the relationship.
- Unresolved Issues:
Lingering issues that have not been resolved can cause resentment, anger, and eventually, the breakdown of the marriage.
Seek Professional Help
If you notice any of the signs mentioned above, it’s time to seek professional help. Many couples delay seeking help until it’s too late, leading to a more challenging and complicated resolution. If you’re wondering whether it’s time to seek professional help, here are some indicators:
- Constant Arguing:
If you find yourself fighting frequently, it’s time to seek professional help. Arguing may be a sign of deeper issues that need to be addressed.
- Withdrawal:
If you or your partner withdraws from the relationship and avoids confrontation, it can be a sign of underlying problems that require professional intervention.
- Lack of Intimacy:
When there is a lack of intimacy or sexual desire, it can be an indicator of deeper issues that need to be addressed.
- Resentment:
Resentment builds up when there are unresolved issues in the relationship. A professional counsellor can help you work through these issues and move forward.
- Loss of Connection:
When you or your partner feels disconnected or disengaged from the relationship, it’s time to seek professional help to re-establish the connection.
How Relationship Counselling Helps
Relationship counselling can be an effective way to resolve issues in your marriage. Here’s how it can help:
- Improves Communication: Counsellors can help couples learn effective communication skills, which can help resolve conflicts and strengthen the relationship.
- Identifies Underlying Issues: Counsellors can identify underlying issues that may be contributing to problems in the relationship and help couples work through them.
- Re-establishes Intimacy: Counsellors can help couples identify barriers to intimacy and develop strategies to improve their connection.
- Develops Healthy Boundaries: Counsellors can help couples establish healthy boundaries and expectations, which can lead to a more fulfilling relationship.
- Develops Problem-Solving Skills: Counsellors can teach couples how to resolve conflicts and find solutions to problems in the relationship.
Conclusion
If you’re noticing any signs of a troubled marriage, it’s essential to seek professional help before it’s too late. It can be a valuable tool in resolving issues and saving your marriage. Don’t wait until your relationship is beyond repair. Seek help today and work towards a happier, healthier relationship. If you are struggling in your marriage and are unsure whether to seek professional help, consider relationship counselling. Don’t let your relationship suffer. Contact a professional counsellor today and take the first step towards a healthier, happier marriage.

Does the gender or age of your therapist matter?
Counseling is a special kind of talk where people share their worries with someone who can help them. Imagine you’re at the Grimsby Counselling Centre, about to meet your new therapist. You might have a big question: “Does it matter if my therapist is a […]
healthCounseling is a special kind of talk where people share their worries with someone who can help them. Imagine you’re at the Grimsby Counselling Centre, about to meet your new therapist. You might have a big question: “Does it matter if my therapist is a boy or a girl, young or old?”
It is an excellent question to ask! It can make a big difference to your therapy journey. You may feel more comfortable with some people than others. Your comfort can change how you share your thoughts and feelings. Let’s explore if the age or gender of your therapist matters.
Understanding the Role of a Therapist Regardless of Age or Gender
A therapist, no matter their age or gender, is trained to provide support and help clients navigate through their mental health issues. They are skilled listeners who use their knowledge to help clients uncover insights about themselves and find ways to cope with life’s challenges.
Exploring Personal Preferences and Comfort Levels
Ultimately, the decision to choose a therapist based on their gender or age is a personal one. It’s crucial to reflect on your preferences and needs and consider how these factors may or may not contribute to your therapy experience.
Engaging in open communication with potential therapists during the initial consultation can help you determine if they fit you, regardless of gender or age. Remember that the therapists primary objective is to support your mental well-being and help you achieve your therapeutic goals.
The Gender of a Therapist Could Influence the Therapeutic Bond
In some cases, the gender of the therapist might matter. For example, someone who has experienced gender-based trauma may prefer a therapists of a specific gender. The aim is to create a safe and comfortable space for therapy.
The Age of a Therapist Might Affect Your Connection
Just as gender can affect the therapeutic relationship, so can the age of a therapists. Younger clients might feel more understood by a younger therapist, while older clients may feel more comfortable with someone closer to their age.
The Therapist’s Competence and Expertise
When considering therapy, it’s essential to prioritize the therapist’s competence, expertise, and qualifications above their gender or age. A skilled therapist will possess the necessary training, experience, and knowledge to address various mental health concerns.
Credentials such as licensure, certifications, and specialization in specific therapeutic approaches or modalities are far more significant indicators of a therapist’s ability to provide effective treatment.
The Final Decision Rests with You
Most importantly, you feel comfortable and safe with your therapist. The age or gender of your therapist can play a role, but it is just one piece of a much larger puzzle. It’s essential to take your time, explore your options, and listen to your feelings as you make this critical decision. Remember, this is about your journey and your healing. And who better to make that choice than you? So, take the first step. Start exploring your options today and find the right therapist for you.

by Randal Spain
Cremation Services in Canada: Exploring Your Choices
Cremation, a practice that dates back centuries, has gained prominence as a preferred method of dealing with a loved one’s remains in Canada. With its growing popularity, the country offers a range of cremation services that cater to various cultural, religious, and personal preferences. This […]
BusinessCremation, a practice that dates back centuries, has gained prominence as a preferred method of dealing with a loved one’s remains in Canada. With its growing popularity, the country offers a range of cremation services that cater to various cultural, religious, and personal preferences.
This article aims to provide a comprehensive overview of the cremation options available in Canada, empowering individuals and families to make informed choices during these sensitive times.
Understanding Cremation Services
Cremation involves the process of reducing a body to its essential elements through intense heat, leaving behind ashes and bone fragments. This method has gained acceptance due to its cost-effectiveness, environmental considerations, and the flexibility it offers in terms of memorialization. Here are some key aspects to consider when exploring cremation services:
- Direct Cremation: This is a basic option where the body is cremated shortly after death without a funeral service. Families can then choose to hold a separate memorial event at their convenience. Direct cremation is often chosen for its simplicity and affordability.
- Traditional Funeral with Cremation: Families can opt for a traditional funeral service, complete with a viewing, followed by cremation. This allows loved ones to pay their respects before the cremation takes place.
- Personalized Memorial Services: Families can personalize a cremation by designing a unique memorial service that reflects the personality and interests of the deceased. This can include music, readings, and even video presentations.
Considering Cultural and Religious Preferences
Rich tapestry of canadian lifestyle and cultures and religions. Cremation services are designed to accommodate a variety of beliefs and practices:
- Christianity: Many Christian denominations now accept cremation as an alternative to traditional burial. The ashes are often interred in a cemetery or placed in a columbarium.
- Hinduism: Cremation holds deep religious significance in Hinduism. The ashes are typically scattered in a sacred body of water, symbolizing the journey of the soul.
- Buddhism: Buddhism often practices cremation as a means of detachment from the physical body. The ashes may be enshrined in a stupa or spread in a serene location.
- Indigenous Traditions: Some Indigenous communities have their own unique practices for handling remains. It’s essential to consult with community elders or leaders to ensure cultural sensitivity.
Environmental Considerations
As the world becomes increasingly environmentally conscious, many individuals are seeking eco-friendly alternatives for end-of-life arrangements:
- Green Burial: While not strictly cremation, green burial involves burying an unembalmed body in a biodegradable container. This option has a lower environmental impact compared to traditional burial.
- Water Cremation (Aquamation): Also known as alkaline hydrolysis, this method uses water and chemicals to break down the body. It’s considered more environmentally friendly than traditional flame-based cremation.
Post-Cremation Options
After cremation, families have several choices for what to do with the ashes:
- Urns: Ashes are often placed in decorative urns, which can be kept at home, interred in a cemetery, or placed in a columbarium.
- Scattering: Families can choose to scatter the ashes in a location that held significance to the deceased. It’s important to ensure that the chosen location allows for this practice.
- Memorial Jewelry: Some companies offer the option to turn a small portion of the ashes into memorial jewelry, allowing loved ones to carry a physical reminder.
Conclusion
In the midst of grief and loss, choosing a cremation service that aligns with your preferences and beliefs can provide solace. Whether you opt for a direct cremation, a personalized memorial, or an eco-friendly solution, Canada offers a range of choices to suit various needs. During these difficult times, it’s crucial to take the opportunity a cremation in a way that pays tribute to the life that was lived.

How Funeral Homes Provide Comfort for Families
Losing a loved one is a deeply emotional and challenging experience. During such times of profound grief, families often find solace and support from funeral homes, which play a vital role in guiding them through the process of honouring and remembering their departed loved ones. […]
BusinessLosing a loved one is a deeply emotional and challenging experience. During such times of profound grief, families often find solace and support from funeral homes, which play a vital role in guiding them through the process of honouring and remembering their departed loved ones.
In Canada, as in many other parts of the world, funeral homes offer not just practical services, but also a compassionate environment that helps families navigate the complex journey of grief and healing going beyond the immediate family, encompassing support for grieving neighbours who seek solace during difficult times.
The Role of Funeral Homes in Providing Comfort
- Emotional Support: Losing a family member or a friend can be an emotionally turbulent experience. Funeral home staff are trained to provide compassionate and empathetic support to grieving families. They offer a listening ear and a comforting presence, helping families navigate the range of emotions they may be experiencing.
- Guidance in Decision-Making: Planning a funeral involves a multitude of decisions, from choosing the type of service to deciding on burial or cremation. Funeral homes offer guidance and information to help families make informed decisions that align with their cultural, religious, and personal preferences.
- Personalization of Services: Every individual is unique, and funeral homes recognize the importance of personalizing services to reflect the life and personality of the deceased. From selecting the right floral arrangements to arranging for meaningful tributes, funeral homes assist families in creating a personalized and memorable farewell.
- Logistical Support: Coordinating a funeral involves numerous logistical details, such as obtaining necessary permits, transporting the deceased, and managing the schedule of events. Funeral homes handle these aspects, allowing families to focus on their emotional well-being.
Coping with Grief: A Multifaceted Approach
- Counselling Services: Many funeral homes offer grief counselling services to help families navigate the complex emotional landscape that follows a loss. These services can be invaluable in providing a safe space for families to express their feelings and receive guidance on coping strategies.
- Support Groups: Connecting with others who are going through similar experiences can be incredibly comforting. Funeral homes may organize support groups that bring together individuals who are grieving, allowing them to share their stories and offer each other encouragement.
- Resource Materials: Funeral homes often provide resources such as brochures, articles, and books that offer insights into the grieving process. These materials can help families understand the stages of grief and provide them with strategies for self-care.
Honouring the Deceased: Rituals and Ceremonies
- Traditional and Cultural Sensitivity: Funeral homes are well-versed in diverse cultural and religious practices. They work closely with families to ensure that the rituals and ceremonies are carried out in accordance with their beliefs and traditions.
- Creating Lasting Memories: Funeral homes assist families in creating meaningful memorial services that celebrate the life of the deceased. From photo displays to video tributes, these services offer a platform for families to reminisce and cherish the memories they shared.
Conclusion
Losing a loved one is a profoundly challenging experience, and during such times, funeral homes offer the vital support needed by families to navigate the journey of grief. Some funeral homes support grieving neighbours to bring them solace during difficult times. Their compassionate professionals provide emotional guidance, practical assistance, and resources for healing for families. If you or a grieving neighbour need support during these difficult times, reaching out to a reputable funeral home can provide the solace and assistance required to honour the memory of your loved one while finding the path toward healing.
In times of grief, it’s important to remember that you’re not alone. Remember, seeking support is a sign of strength during times of vulnerability. Let funeral homes help you and your family through this period, offering comfort and assistance every step of the way.

The Benefits of Hiring a Local Criminal Defense Attorney
When facing criminal charges, having a competent legal professional by your side is crucial. Hiring a local criminal defence attorney offers numerous advantages that can significantly impact the outcome of your case. In-depth Knowledge of Local Laws Local criminal defence attorneys comprehensively understand the laws […]
Law & LegalWhen facing criminal charges, having a competent legal professional by your side is crucial. Hiring a local criminal defence attorney offers numerous advantages that can significantly impact the outcome of your case.

In-depth Knowledge of Local Laws
Local criminal defence attorneys comprehensively understand the laws specific to your jurisdiction. They are well-versed in the intricacies of the Canadian legal system and have experience handling cases in your local courts. The knowledge of such bail hearing process allows them to navigate the legal processes effectively and craft a tailored defence strategy for your situation.
Familiarity with Local Court Procedures
Each court system has its unique set of rules and procedures. By hiring a local criminal defence attorney, you gain access to their familiarity with the local court system. They understand the requirements for filing documents, deadlines, and courtroom decorum. This familiarity helps to ensure that your case proceeds smoothly and efficiently through the legal process.
One enjoys Personalized Attention and Availability
Local criminal defence attorneys typically have smaller caseloads than larger firms. This allows them to dedicate more time and attention to each client. When you hire a local attorney, you can expect personalized service and direct access to your lawyer. They will be readily available to answer your questions, address your concerns, and keep you informed about the progress of your case.
Enjoying Local Networks and Relationships
Local criminal defence attorneys often have strong professional networks and relationships within the legal community. These connections can be invaluable when building your defence. Your attorney may have established relationships with judges, prosecutors, and other key figures in the legal system. This familiarity can help them negotiate effectively on your behalf, potentially leading to favourable outcomes or reduced charges.
Having substantive Knowledge of Local Resources
Local criminal defence attorneys are well-acquainted with the resources available in your community. They can connect you with local experts, investigators, or specialists who can provide valuable insights or testimony to support your case. Additionally, they may be familiar with local rehabilitation programs or alternative sentencing options that could benefit your situation.
Understanding of the Local Community
Hiring a local criminal defence attorney means working with someone who understands your local community’s unique characteristics and dynamics. They know the social, cultural, and economic factors that may impact your case. This understanding enables them to build a defence strategy that considers the specific context of their situation, increasing their chances of a positive outcome.
Hiring a local criminal defence attorney in Canada offers several advantages. Their in-depth knowledge of local laws and court procedures, personalized attention, professional network, access to local resources, and understanding of the community all contribute to a stronger defence for you. When facing criminal charges, it’s essential to have a competent and experienced advocate by your side, someone intimately familiar with your area’s legal landscape.
If you’re facing criminal charges in Canada, don’t underestimate the importance of hiring a local criminal defence attorney. With their knowledge, experience, and connections, they can provide you with the best possible defence. Take action today and consult with a local attorney who will fight for your rights and protect your interests.

How Courier Services Saving Time and Money
Courier Services are exceptional companies. They help us move packages from one place to another. They’re like mail services but faster and more personalized. These services are used for business or personal needs. They are helpful when we need to send things quickly. Why Use […]
BusinessCourier Services are exceptional companies. They help us move packages from one place to another. They’re like mail services but faster and more personalized. These services are used for business or personal needs. They are helpful when we need to send things quickly.

Why Use Courier Services?
Courier service can deliver the following benefits compared to other package/document delivery options:
- They are fast and reliable.
- They offer door-to-door service.
- They have tracking systems.
- All these benefits save you time and money.
How Do Courier Services Save You Time?
Courier services offer Fast Delivery
The first thing about courier service is their speed. They are much faster than regular mail services. Standard mail can take several days. Pack a parcel for a courier can deliver on the same day or the next day. This quick service means you spend less time waiting for your packages.
Courier services offer Door-To-Door Service
Another great thing about courier service is the door-to-door service. You don’t have to go anywhere to send or receive packages. The courier comes to your location. They pick up the box and deliver it to the receiver’s location. This service saves you time. You don’t have to spend time traveling to the post office.
Courier services offer Tracking System
Courier services offer tracking systems. These systems let you know where your package is. You can track your parcel from when it leaves you until it reaches its destination. This saves you time and stress. You don’t have to worry about where your package is.
How Courier Services Save You Money
No Need for a Vehicle
Using courier service means you don’t need your delivery vehicle. This can save you a lot of money. Vehicles are expensive. They need fuel. They need maintenance. With courier services, you don’t have to worry about these costs.
Save on Manpower
Another way courier service save you money is by reducing the workforce. If you had to deliver packages yourself, you would need to hire people. These people would need salaries. They would need benefits. Courier service take care of this for you. They have their people. They take care of the delivery.
Reduced Risk of Lost Packages
Courier service have insurance. They cover the cost of lost or damaged packages. This saves you from potential losses. Regular mail services may offer different coverage. If they lose your package, you lose money. With courier services, you have more security.
How to Choose the Right Courier Service
Choosing an exemplary courier service is essential. It can save you even more time and money. To make the best decision, consider the following advice.
Compare Rates
Different courier services have different rates. Some may charge more for the same service. It’s crucial to compare rates before choosing a courier. This way, you can get the best service at the best price.
Check Reviews
Reviews can tell you a lot about a courier service. They show what other people think about the service. Look for a courier service with good reviews. Good reviews mean good service. It means the courier is reliable and worth your money.
Consider Their Experience
Experience matters in courier services. The more experience a courier has, the better. Experienced couriers know how to handle packages well. They know how to deliver them quickly and safely. They are less likely to lose or damage your packages. You may avoid a lot of headaches and expenses by doing this.
Some Final Words on More Cost-efficient Courier Services
Courier services are a great way to save time and money. They offer fast, reliable, and secure delivery. They save you from having to buy a vehicle or hire people. They offer insurance to protect you courier from losses.
But remember, not all courier service are the same. You need to choose the exemplary courier service to get the most benefits. It would help if you compare rates, check reviews, and consider their experience in delivering the results you’re looking for.
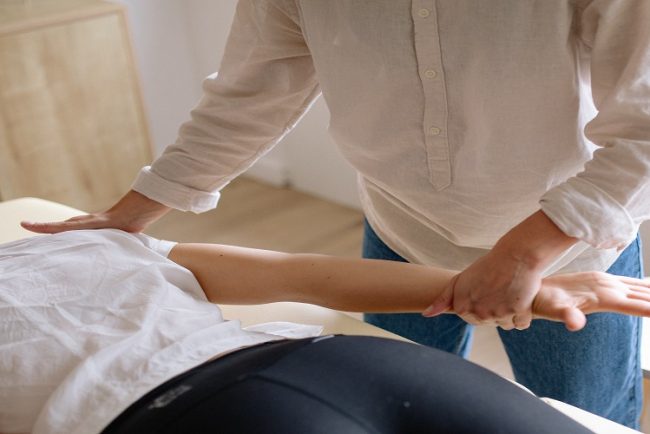
How Physiotherapy Can Help with Chronic Pain Management
Chronic pain is a common and complex health issue that affects millions of Canadians. Chronic pain is a type of pain that persists for several months or even years, affecting an individual’s quality of life, work productivity, and emotional well-being. Managing chronic pain can be […]
healthChronic pain is a common and complex health issue that affects millions of Canadians. Chronic pain is a type of pain that persists for several months or even years, affecting an individual’s quality of life, work productivity, and emotional well-being. Managing chronic pain can be challenging and often requires a multidisciplinary approach that includes medication, exercise, and lifestyle changes. One effective method that has gained popularity in recent years is physiotherapy.
In this article, we will explore how registered physiotherapists can help manage chronic pain and improve an individual’s overall quality of life.
Understanding Chronic Pain:
Chronic pain is a complicated condition that can result from various causes such as injury, surgery, or underlying medical conditions like arthritis or fibromyalgia. Unlike acute pain, which typically lasts for a short period, chronic pain can persist for months or even years, causing discomfort, fatigue, and emotional distress. Chronic pain can affect an individual’s physical, emotional, and social well-being, leading to reduced mobility, depression, and anxiety.
How Can Registered Physiotherapists Help?
Registered physiotherapists are healthcare professionals who specialize in treating conditions that affect an individual’s movement, function, and overall well-being. They use evidence-based techniques to help individuals manage their chronic pain and improve their quality of life. Here are some ways that Physiotherapy can help:
- Assessment:
Physiotherapy will conduct a comprehensive assessment of an individual’s condition to determine the underlying causes of their chronic pain. The assessment may include a physical examination, medical history, and functional assessment to identify areas of weakness, stiffness, or muscle imbalances.
- Education:
Registered physiotherapists will provide individuals with education about their condition and how to manage their symptoms. They will teach individuals about pain management techniques, proper posture, and ergonomics to reduce the risk of further injury or exacerbation of symptoms.
- Exercise Prescription:
Registered physiotherapists will develop a personalized exercise program for individuals that focus on improving their strength, flexibility, and endurance. Exercise can help reduce pain, increase mobility, and improve an individual’s overall quality of life.
- Manual Therapy:
Registered physiotherapists can use various manual therapy techniques like massage, joint mobilization, or soft tissue release to reduce pain, improve circulation, and restore mobility. These techniques can help reduce pain and improve an individual’s range of motion.
- Modalities:
Registered physiotherapists can use various modalities such as ultrasound, electrical stimulation, or hot/cold therapy to manage an individual’s pain and promote healing.
Conclusion:
Chronic pain can be a debilitating condition that affects an individual’s quality of life. Best Physiotherapists can provide individuals with education, exercise prescription, manual therapy, and modalities to manage their pain and promote healing. If you are experiencing chronic pain, consider consulting a physiotherapist to help you manage your symptoms and improve your quality of life. If you are struggling with chronic pain, consider consulting a registered physiotherapist to help you manage your symptoms and improve your overall quality of life. Contact us today to book an appointment with one of our registered physiotherapists and start your journey toward pain-free living.
More Stories


7 Questions You need to ask during Denture consultations
Whether you are seeing a dentist for the first time or not, nearly all dental procedures begin with a denture consultation. It is the first and most essential step to getting what you want from the dentist. Whether you book a free consultation or a paid […]
healthWhether you are seeing a dentist for the first time or not, nearly all dental procedures begin with a denture consultation. It is the first and most essential step to getting what you want from the dentist. Whether you book a free consultation or a paid one doesn’t matter. Preparing for this consultation beforehand will help you get the most out of it. This post shares seven crucial questions to ask during a denture consultation.
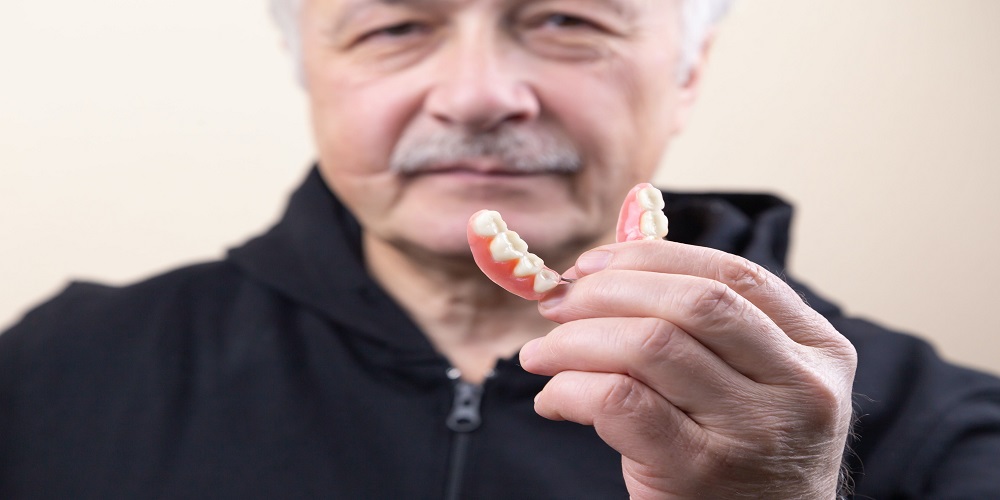
What dental procedure do I need?
It is great that you have decided to improve your denture or oral health. You may have thought about different procedures and wondered if they were right for you. One effective way to get what you want from a consultation is by explaining your goals to the professional. Then ask them to recommend the best procedure to achieve that goal. For example, you might think of dentures when dental implants or veneers are better for you. The dentist will recommend the best procedure.
How long have you been doing the procedure for patients?
The best dentist started somewhere. But experience matters when getting a dental procedure. What they learned in dental school is important. But it would be best if you also cared about the experience. It ensures the dentist can handle your surgery, no matter how complex. A few years in the area where you need their service would increase your confidence in the dentist.
What are the risks of this procedure?
The dentist should explain any risks associated with what they’ve recommended. Would it be painful? If so, how would the pain be managed? Knowing the risks helps you decide whether the procedure is worth it.
How long should I expect the result to last?
Many dental procedures do not last forever. Some have specified time frames for which a renewal is required. Remember that you’re investing money in the service. Therefore, find out how long the investment will last before you enter into it.
What is the recovery process like?
Every recovery process depends on the work that was done. Some may disrupt your daily routine for a while. Ask the dentist about diets or meds that can promote fast recovery. This information also prepares you mentally for the procedure.
What does my insurance cover?
There are different dental insurance. Their coverages differ. Even if the dentist does not have the answer, they can connect you to someone who does.
Do you have payment plans?
Dental insurance covers some dental work. But even those require paying a part of the bill. If you are unable to make the payment at once, you can ask the dentist about payment plans. Many have affordable financing options and plans to ease the process.
It is possible and easy to achieve optimal dental health. One way to do that is to be prepared before any session with your dentist, first appointment, or others. With these questions, you can maximize any denture consultation whether it is free or paid.

How to Apply Makeup
To make your makeup look its best, you must first know how to clean and moisturize your face. A properly cleansed and moisturized face will be smooth and free from flakes and texture. Using the right makeup products will make your face look its best! […]
Beauty & FashionTo make your makeup look its best, you must first know how to clean and moisturize your face. A properly cleansed and moisturized face will be smooth and free from flakes and texture. Using the right makeup products will make your face look its best! To make your makeup look its best, follow the following steps:
Layering technique helps blend makeup
The layering technique is one of the most popular techniques used by makeup artists. This technique works by applying the foundation and other products in layers. Using a blending brush, you can blend the first layer into the second and even out the look. You should then repeat the process with the last layer. Then, blend in any extra product with the same tools you use on the other layers. Using the layering technique will create the perfect makeup base that will last all day.
Primer helps your makeup glide smoothly
Before applying face makeup, make sure that you use a primer to ensure a smooth glide. It works to protect your skin, minimize fine lines and pores, and helps your makeup glide on smoothly. Apply primer in the center of your face and blend it out using gentle circular motions. Apply it to your hairline, neck, and skin around the eyes as well. This will help the makeup stay put, as well as prevent your foundation from sliding off.
Concealer helps conceal dark circles
Applying a corrector under your eyes before concealer helps cover up dark circles when applying makeup. Dark circles tend to be blue or gray in color. A concealer that covers up this discoloration should have a peach or orange tone. Choose a shade two or three shades darker than your skin tone. By applying concealer in a triangle with your ring finger, you can hide your dark circles.
Blush helps highlight cheekbones
Apply blush on the upper side of your cheekbones, just above the hollow beneath them. Blush helps bring out your cheekbones and balance out the width of your chin and forehead. Use the same shade of blush to contour the hollows of your nose. For the best results, apply blush on the cheekbones as you would a contour, using a long, light motion. Applying blush on the cheeks is easier when you’re smiling, as it will make your cheekbones pop.
Lip gloss helps brighten lips
You can add a little radiance to your pout with the right lip gloss color. Glossy lips look fuller and more appealing, so go for a lighter color when applying makeup. For fair-skinned women, the best options are dedicated and spirited. You can also use the applicator that comes with the gloss to apply the color on your lips. For maximum shine, you can use two or three coats of the product.
Using a makeup sponge
When it comes to applying makeup, nothing compares to the smooth, even finish that a good quality makeup sponge provides. To maintain the beauty of your makeup sponge, you should wash it regularly with a mild soap or cleanser. You should massage the sponge thoroughly to remove any excess makeup, and then rinse it under running water. Once the sponge is clean, simply lay it flat to dry. Ideally, you should replace your makeup sponge every two to three months, depending on how often you use it.
Using a brush
Whether you want to create a flawless-looking complexion with a matte finish or a shimmery sheen, using a brush to apply your foundation is a must. The correct application can leave you with skin that looks smooth and flawless, and can ensure you get the coverage you want without streaking or smearing. Here are some tips for choosing a brush. Use an oval brush for foundation.
Using a translucent powder
Unlike tinted face powder, a translucent powder is a white formula that applies without any color or coverage. It’s used to minimize shine, absorb oil, and brighten the skin. Because of its brightening properties, it’s great for under the eyes, the center of the chin, and around the nose. The powder can even be used as a concealer. Use translucent powder sparingly on the eyes, chin, and nose.

House Plants That Don’t Die Easily
If you’re looking for some easy houseplants that don’t die easily, there are many great choices. These include African spear plant, Peace lily, and Red rubber tree. Read on to learn more about each. And remember: don’t be afraid of trying something new. Try out […]
Home ImprovementIf you’re looking for some easy houseplants that don’t die easily, there are many great choices. These include African spear plant, Peace lily, and Red rubber tree. Read on to learn more about each. And remember: don’t be afraid of trying something new. Try out a new plant every year! It might surprise you! You might also like these unusual plants:
Red rubber tree
The Red Rubber Tree is one of those easy house plants that don’t die easily. Rubber plants are moderately tolerant of salt. They sprout several stalks at once. To encourage multiple plant growth, prune the plants every year. In order to keep them looking beautiful, use a wet sphagnum peat moss and root hormone solution when pruning. After pruning, keep the plant moist and move it into a larger planter every one or two years.
When watering your Red Rubber Tree, make sure that you do not drown it. If it dries up too quickly, the leaves may look tangled or yellow. This could be caused by too much or too little water. Make sure to read the watering guide and follow cultural practices. If you notice any brown spots, they may be caused by overwatering or bacterial infection. In order to avoid this problem, you should keep the plant in a partially shaded area, if you want it to look its best.
Dracaena
If you are looking for a plant that won’t die easily, a dracaena might be the perfect choice. Although it is tolerant of drought, it still needs regular watering, particularly if the top inch of soil is dry. But avoid overwatering, which can result in root rot. Another cause of browning tips on dracaenas is low humidity. A humidifier or a tray of water can help maintain humidity. If you live in an area with hard water, you can use filtered water or leave the tap water out overnight.
The good news is that dracaenas are surprisingly easy to care for. Besides being an excellent plant for indoors, dracaenas can even be propagated, so you don’t need to worry about wasting your money on expensive nursery plants. To propagate dracaenas, you can simply cut their stems and transplant them into larger containers.
African spear plant
The African Spear plant requires very little care to thrive. This plant can live for many years with minimal maintenance. In fact, many experienced gardeners do not discard its dried leaves. Instead, they use them for various crafts, such as weaving or crocheting. Besides, it provides a constant supply of fresh air to the room. You can use it as a living air purifier if there is a stale smell in the house.
African Spear plants prefer bright indirect sunlight. They will tolerate a little bit of direct sunlight, but need at least four to six hours of bright light a day. For optimal growth, plant them near a west or east-facing window. During the hottest part of the day, move them to a shady spot. They are toxic to children and pets, so if you are unsure, don’t let them play around with the plant.
Peace lily
If you’re looking for one of the easiest house plants that don’t die easily, a Peace Lily is probably what you’re looking for. This plant doesn’t require much maintenance, but you should be aware of its sensitivity to certain conditions, such as excessive watering or fertilizer. Occasionally, peace lilies will show signs of leaf yellowing. If you notice this behavior in your peace lilies, you may want to take action.
To propagate peace lilies, you can divide the plant. First, dig out the mother plant. Make sure you don’t pull the roots too quickly, as this will result in poor propagation. Look for a clump of roots with several leaves. Carefully cut these roots off and plant the new crown in the same pot. Once you have the new plant, don’t fertilize it for three months, as it might burn the new plant.
Aloe vera
If you’re considering adding a house plant, consider starting with aloe vera. It produces offsets (plantlets or pups) that will gradually fill the pot. You can also separate the pups and plant them separately, or gift them to a friend. Aloe Vera propagation is very easy. Simply divide the plant and propagate the offsets. These plants can grow very large over the years if given the proper care and conditions.
The aloe needs bright natural light to thrive. It can survive up to six hours of direct sunlight, but should receive some respite from the sun in the afternoon. It’s best to keep it near a sunny window. Direct sunlight will burn its tender skin. Lack of light will weaken its leaves and cause it to grow leggy. A well-lit window will allow it to thrive.

Why You Should Start an Online Business in 2022
In this article, we’ll examine some of the key factors that influence the success of an online business. In order to succeed, you need to solve a real problem. In the world of fashion, for example, people want to stand out from the crowd without […]
BusinessIn this article, we’ll examine some of the key factors that influence the success of an online business. In order to succeed, you need to solve a real problem. In the world of fashion, for example, people want to stand out from the crowd without spending a lot of money. By creating a stylish clothing line that is both affordable and flamboyant, you’ll be able to attract a targeted audience.
In the past, only the most privileged people could launch a successful business. Starting capital was high and outside funding was limited. However, in 2021, any person could start a successful online business. While the entry barrier has lowered significantly since 2000, many of the same factors still apply – you’ll need a solid product idea, a sourcing and manufacturing strategy, and a sales model.
The pandemic, for instance, has completely transformed the way people shop. Even those who can’t shop in person have gotten on board the e-commerce bandwagon. The statistics show a 40% increase in e-commerce since the outbreak. With this in mind, 2022 is a great time to start an online business. So, if you have a great business idea, you should definitely start it in 2022.
Before you start your online business, you’ll need to research the legal requirements, which vary from state to state. Remember that you’ll need to be a sole proprietor to start your business, so you should check with a tax specialist to ensure you don’t run afoul of the law. In addition, some businesses require licensing or registration. Again, research will be your best guide in determining whether you’ll need a license.
There are numerous opportunities for funding your online business. The US Chamber of Commerce has compiled a list of 49 funding options. Click through to get the details about each one. The list also includes information on how to apply for each one. While some of the funding options will require a large upfront investment, some will require minimal effort. However, you’ll need to shop around to find the right partnership for your online business.
Online businesses require a website. Using a website builder, you can test a business model and gain your first clients. Once you have your first clients, you can begin monetizing your online store. You can even use a DIY website to get a website up and running without hiring a web designer. For less, you can use an affordable, fully customizable template like Boxmode. In the end, online businesses require no capital.
Listed below are some examples of products that can make you money. Many of these ideas are already in existence in existing industries. They are just missing the right product or service to meet their needs. They can also be sold as a digital product on marketplaces. The key is to create a strong online presence in your niche and make sure it is relevant to your target audience. The more you know about your market, the better.
Another benefit to starting an online business is that you can control your own schedule. You can plan your own hours and work around your regular lifestyle. You can do what you want when you have time. Unlike a traditional business, there are no pre-registration requirements. Moreover, online businesses do not require you to pre-register. You can work around your family or other commitments. It’s a great way to earn extra money.
If you’re interested in starting an online business, a solid niche that will still generate substantial profits is the fitness and nutrition industry. Fitness-focused businesses will do well to sell meal plans and workout plans. They can also offer digital products or even e-books. Those interested in marketing can also start an online advertising business. You can also offer your services to local businesses. If you’re good at this, you could even consider working with them.

Why You Should Hire a Personal Fitness Trainer for the Gym
The gym is an unwelcoming place for people who aren’t particularly committed to their workout sessions. It can be difficult to get motivated to get up and go, and a personal trainer can give you the extra motivation you need to stick to your workout […]
Beauty & FashionThe gym is an unwelcoming place for people who aren’t particularly committed to their workout sessions. It can be difficult to get motivated to get up and go, and a personal trainer can give you the extra motivation you need to stick to your workout routine. The main drawback of working out alone is that it can quickly become monotonous. Personal fitness trainers make working out a fun experience, and they can motivate you to keep going if you’re bored.
Hiring a personal fitness trainer can have a number of benefits, from teaching you how to perform a specific exercise to helping you achieve your goals. A personal trainer can also help you develop a routine that works for you, including setting specific times to meet with him. This helps you build a positive fitness habit and stay consistent throughout your workout. Personal trainers are also skilled at creating custom workouts that cater to your fitness goals, including introducing new forms of exercise.
Hiring a personal fitness trainer for the gym is an excellent choice for those looking for motivation and accountability. If you’re the type of person who struggles to stick to a schedule and is concerned about achieving fitness goals, a personal trainer will encourage you to persevere. Having a friend or coworker guide you through your workouts is also an excellent idea. However, personal trainers are more costly than private gym sessions.
Another reason to hire a personal fitness trainer is to ensure that your routine is challenging enough to reach your goals. Moreover, hiring a personal trainer can introduce you to new exercises that will keep your workouts fun and fresh. A personal fitness trainer can also develop multiple workout routines for you, including bodyweight exercises, free weights, cardio, and other types of exercise. This can help you achieve your goals more quickly and safely.
How to be comfortable with a personal fitness trainer
A fitness trainer’s personality and training style go hand-in-hand. You might be fast on the uptake of new skills, but if you tend to complain and slack off during workout sessions, you might wind up irking your personal trainer. To avoid misunderstandings, try to get to know your personal trainer as well as your gym. Then you can both agree on your personal goals and get started!
During your first meeting, be sure to tell your personal trainer everything you want to know. This includes any personal details you want to disclose, like your goals. A good trainer will take notes during the interview, and they will never body shame or make you feel uncomfortable. They should also be willing to listen to your goals and give you a workout tailored to your needs. It helps if the trainer has a pleasant personality and you feel relaxed with them.
If your gym is new to you, be sure to meet your trainer before committing to a monthly plan. The first session is an opportunity to meet your trainer and gauge whether you will get along. Often, a trainer will get a feel for you based on his or her approach, attitude, and vibe. It may take the entire initial session before you know if you click.
How long a personal fitness trainer lasts
A typical personal training session lasts between 30 and 60 minutes. A personal trainer will measure your body and discuss your past health and exercise history to tailor your workout to your goals. The trainer will show you proper form for exercises, help you figure out how much weight to use, and provide pointers on how to get the most out of your workout. Typically, a session lasts between one and three months.
At Equinox, the average personal trainer turnover rate is 50 percent per year, which is high for this industry. The gyms also declined to reveal the turnover rate for their beginner trainers, which comprise about 10 percent of the staff. In recent years, the personal training industry has become the fastest-growing occupation in the United States, with celebrities sharing their training tips on social media. However, some gyms don’t pay personal trainers to recruit clients.
Another consideration is the time of day. Some trainers work during slow periods, such as Thursdays and Fridays. This is because most people are out socializing on Friday evenings, so they’re less likely to work out on these days. However, Saturday and Sunday mornings are the busiest times of day for fitness centers, so it’s important to consider when the best time to work is.

Essential Items to Take When You Go on Holiday
You may need a few things when you are traveling, but you should never leave your house without a few essential items. Here are a few items that you must have in your holiday bag. Ensure you take enough of these items, or they may […]
AutoYou may need a few things when you are traveling, but you should never leave your house without a few essential items. Here are a few items that you must have in your holiday bag. Ensure you take enough of these items, or they may be too large for your suitcase and are useless. Before you go on holiday, make a list of essential items. You must remember to include all items that you may need while you are traveling, but don’t forget to bring a travel health bag with you.
First of all, a first aid kit is an absolute must, because you never know when an unexpected medical emergency will strike. In addition to a first aid kit, a travel kit should also contain a few antiseptic wipes and first aid supplies. Children can get a bit bored on long trips, so bring along a travel pillow, books, or their favorite bedtime stories. They can also play games on their phones.
In case of a medical emergency, make sure to pack your medication. Don’t forget to pack a variety of sun creams with various SPF factors, and don’t forget your toothbrush and aftersun. If you plan to travel by car, remember to carry your licence as well as photo ID. The airport currency exchange rates may not be as good as other places. If you have an active lifestyle, pack plenty of sunscreen and insect repellent. Also, don’t forget to check on vaccination requirements before you leave.
Finally, sunglasses are essential items to bring on holiday. No matter where you’re travelling, sunglasses are a must. They keep you safe from the sun, which can be harmful to your eyesight. You can purchase an inexpensive pair for the beach. It’s also a good idea to wear a comfortable pair of shoes. You’ll be glad you did. This list may help you decide which essential items to pack for your next vacation!
Some of the other essential items to bring along with you include a sunscreen and a sun cream. For a beach vacation, you should also take a sheer water-resistant makeup formula that contains antioxidants and skin-nourishing ingredients. Another essential item to take on holiday is shampoo and a hair treatment kit. Natural shampoos can help repair damaged hair. They also smell good, so you should make sure to pack a few of these.
If you’re a bookworm, you may want to take along a travel language book or phrasebook. You might even want to bring along a deck of playing cards for some fun on the road. And remember to pack enough clothing for your entire holiday. Try not to overpack your suitcase; some hotels will offer laundry services, so you’ll be able to wash a few items. Remember to pack plenty of underwear, too! Also, dress appropriately for the climate you’ll be in.
Lastly, be sure to pack your travel documents. Your travel documents are important, especially if you’re going to be traveling overseas. If you can’t find yours, consider emailing scanned copies of them so they’re not lost. And don’t forget your money! Don’t forget to pack your credit cards, money, and other essential items. Your wallet and purse should be as full as possible, but not stuffed to the brim.
For the duration of your trip, it’s vital to have the right items for the climate you’re traveling to. Summers in North America are hot and humid, so make sure you pack sun protection. It’s always a good idea to pack a hat and sunglasses for protection against the sun. Taking a camera on your holiday is a great way to document your adventures and share them with friends and family back home.
While packing for a holiday, don’t forget your passport and travel money! Although the currency is different from ours, most places will accept American dollars. If you’re traveling to Mexico for example, you should pack your ID, too. This is important because you may need it to cross the border. Having a passport or other form of identification is also recommended. If you’re going to be swimming a lot, make sure you pack sunglasses as well.

Which Country Has Had the Most Tourism in 2022?
If you are wondering which country has had the most tourism in 2022 after the COVID lock downs and restrictions, you are not alone. Greece, Italy, and Turkey are also high on the list. But which one is the most popular? This article will give […]
AutoIf you are wondering which country has had the most tourism in 2022 after the COVID lock downs and restrictions, you are not alone. Greece, Italy, and Turkey are also high on the list. But which one is the most popular? This article will give you all the information you need to make your decision. So, read on to learn more about these countries. In addition to Spain, you may want to consider traveling to Greece or Turkey. These destinations are sure to delight you.
Spain
The Spanish economy has recovered from the recent pandemic and the Iberian islands have become popular summer party locations. With summer holidays fast approaching, Britons are gearing up to head south to the sun-drenched shores of Spain. But recent headlines have suggested that the traditional tourist experience in the country is about to change. In this article, we’ll look at the reasons for this dramatic change.
The Spanish government expects the tourism sector to recover by 2022 after the outbreak of the Omega coronavirus, which ravaged the country last year. This is good news for Spain’s travel industry, as fewer restrictions on COVID-19 will allow more British holidaymakers to visit. Normally, the country attracts around 18 million tourists each year. Until the outbreak of the virus, Spain was the second most visited country in the world after France, but it has suffered a resurgence in recent years due to the emergence of the COVID variant.
Greece
With wildfires and a COVID pandemic causing some countries to impose travel restrictions, Greece has loosened its rules for tourists. No longer do you have to present your Covid recovery certificate or vaccination papers when you visit the country. Only valid travel documents are required. Most restrictions have been removed, however, including the requirement to wear a mask in outdoor or indoor areas.
With more than a quarter of the population relying on tourism, Greeks are well aware of the upcoming winter. After all, the country’s inflation hit a 28-year high in August and minimum wages are low. Nearly half of the labour force can’t afford to take time off for a holiday, so it’s clear that Greece needs a boost in tourism.
Italy
In the latest World Travel & Tourism Council (WTTC) yearly highlights report, Italy was ranked second with a 2.7 percent growth in international visitor arrivals. The country is currently home to 51% of the world’s tourists, a figure that will more than double by 2022. While the rest of the world may have increased its tourism numbers, Italy remains one of the most cautious. While it is still one of the most conservative countries in Europe, recent changes have lowered travel restrictions. No longer do travelers need to prove their vaccination, and they are no longer quarantined upon arrival if they are not.
A number of factors contribute to Italy’s ranking. First, it has a variety of attractions. A country’s cultural diversity is an important factor, with more World Heritage Sites than any other place. Secondly, it offers a diverse landscape. Its icy Alps and turquoise coral reefs are just some of the many reasons to visit Italy. It is also known for its culinary delights, including the delicious ricotta and oysters.
Turkey
Tourism in Turkey is booming despite the recent currency crisis. Last year, it hosted almost 50 million visitors and 25 million of them came to Antalya, a resort town offering 300 days of sun. According to the World Tourism Organization, the number of visitors to Turkey increased by 4 percent over the previous year. France was the most popular destination in the world with 90 million visitors, followed by Spain and Mynmar. Iran and Uzbekistan were the fourth and fifth most popular in the world, while Egypt and Montenegro rounded out the top ten.
Some of the most popular tourist spots in Turkey include the Blue Lagoon, which is surrounded by turquoise waters. Aspendos, Perge, and Manavgat Waterfalls can all be reached via boat and offer a great deal of beauty. The ruins of the former Armenian capital Ani are also a major draw for tourists. Many of these destinations have luxury hotels, and they will make your holiday one to remember!
Thailand
Following the reopening of several Asian countries, Thailand is the leading tourism destination with its relaxed entry requirements, convenient visa policies, and dazzling tourism charms. Fully vaccinated visitors can enter Thailand without a visa; those who aren’t must provide proof of a negative RT-PCR or ATK test within 72 hours of their departure. Visa-free entry is available to 56 countries, and for those whose countries don’t qualify, it offers Visa-on-Arrival.
Thousands of international travelers flock to Thailand each year. The country’s many attractions entice visitors from all over the world. Popular attractions include the Grand Palace in Bangkok, ornate Buddhist wat Coi Suthep temple, and beaches. Visiting the country’s national parks is also a top reason for tourists to visit Thailand. Whether it’s a beach vacation or a cultural getaway, Thailand has something to offer everyone.
Maya Bay on Ko Phi Phi has experienced a sustained explosion in tourism
The impact of mass tourism on Koh Phangan’s Maya Bay is already apparent. The popular beach is attracting 3,800 visitors per day, with most arriving by speedboat from Phuket. But the situation is about to get even worse. In order to reverse this situation, authorities have put a stop to the expansion of tourism in Koh Phangan by limiting visitors to 380 per hour. Visitors are also forbidden to swim in the bay. The only way to reach the beach is to disembark from the boat pier at nearby Loh Sama Bay and walk from there. In April, the island was “flooded” with water during the Songkran holiday. It also closed for two months during August and the low season.
The island is one of the most famous in the world, thanks to the movie “The Beach.” It has no shortage of tourists – in fact, the cove was featured in the film. However, the beauty of Maya Bay has been preserved, despite the increasing number of visitors. The beach has a cliff and a creek where swimmers can jump. To get to Maya Bay, you can take a longtail boat, which costs approximately 1,500 baht per half-day, and the best time to visit is before 8:00am.

Cheap Ideas to Make Your Garden Pretty
You don’t need expensive furniture to decorate your garden. Try adding a few cheap ideas. You can use a Kilner jar to power solar-powered lights, or hang some colourful fairy lights from the trees or bushes. You can even create decorative ladder shelving by tying […]
Home ImprovementYou don’t need expensive furniture to decorate your garden. Try adding a few cheap ideas. You can use a Kilner jar to power solar-powered lights, or hang some colourful fairy lights from the trees or bushes. You can even create decorative ladder shelving by tying two ladders together at the top. You can then lay wooden planks across the runs, forming shelves, and display decorative items. A decorative ladder is an inexpensive and easy way to add a personal touch to your garden.
Bloom boxes
If you don’t have a green thumb, don’t fret, there are many ways to add color and life to your garden for a low cost. A simple bloom box can be constructed from wood, and it won’t cost much to make one. Place one outside the window and you’ll have a lovely garden without overpowering the rest of your landscape. Or, add one inside a flower box and enjoy its beauty all year round.
Container gardening
A giant ice cream cone is a favorite treat for the group of friends. The same thing applies to a giant ice cream box. The soil in this container may dry out quickly if it is left in the sun, so place it in the shade. Another cheap idea for a container garden is to repurpose a kitchen colander. You can spray paint it with an outdoor spray paint and add some landscape fabric to prevent the soil from falling through the holes. You can also reuse a malfunctioning drawer for a container garden.
Bird feeders
One of the simplest and cheapest ideas for attracting birdlife is to install a bird feeder. Pinecones make wonderful bird feeders. Simply collect them on your daily walks and rinse them off when you get home. Next, tie a string to the top of the pinecone. Once the string is tied, spread a small amount of peanut butter on the outside. Place some seeds on top of the peanut butter.
Crushed stone planters
You can use crushed stone planters for a garden on a budget. They are great for filling large gaps in your garden. They also come in a variety of textures and colors, making them a great way to spruce up bare spots. Here are some other tips for making your garden look prettier for less money. Using crushed stone planters is easy and cheap, so get started today!
Recycling old furniture
When you’re planning to decorate your garden or backyard, you can use recycled items to dress it up. Old furniture can be recycled into pretty garden accessories and can be used as a side table or plant stand. Old milk jugs make a stylish plant or flowerpot stand. Recycled milk jugs can be painted and personalized for a unique look. You can also turn a steel drum into eco-sustainable furniture.
Planting perennials
You can buy perennials whenever they’re on sale, or plan your flower bed and purchase them at the right time. If you don’t know where to begin, reading the tags on flower containers can be difficult, and planting them in the correct location may require a fair amount of guesswork. To avoid this problem, make notes of the areas of your garden that need colour now. If you buy perennials in full bloom now, you’ll avoid guesswork and purchase them in time.
Adding a privacy screen
Besides the obvious privacy benefits, a privacy screen can also be an eye-catcher. Bamboo privacy screens are both beautiful and cheap. Bamboo privacy screens are also sturdy and provide a natural, stylish look. For added privacy, install a private rock screen. The combination of the natural beauty of rock and privacy is quite delightful. This will not only make your garden look more natural, but will also provide a good amount of light.

How to Save Money in 2022-2023
If you’re wondering how to save money in 2022, you’ve come to the right place. Many families spend 30-40% of their income on housing. You can reduce your housing costs by moving to the suburbs, purchasing a tiny house, or moving in with family. But […]
BusinessIf you’re wondering how to save money in 2022, you’ve come to the right place. Many families spend 30-40% of their income on housing. You can reduce your housing costs by moving to the suburbs, purchasing a tiny house, or moving in with family. But how do you save money in 2022 without giving up your dream home? Here are some simple ways to save money today. Follow these tips and you’ll be able to retire with plenty of money to spare in 2022.
Simple ways to save money
In 2022, a spending freeze will be a great idea. You can cut costs on the food you already buy or prepare meals using items you already have. Avoiding stores where you tend to buy on impulse is also an excellent way to cut costs. Simply saying “no” to unnecessary purchases can save you a bundle! Try out these simple ways to save money in 2022 and begin feeling more content with your financial situation.
Stop using subscriptions. Cable TV subscriptions cost around $217 per month. That’s not even counting the fees. This can add up to $2,600 a year. Consider using network apps or streaming services instead. Try to subscribe to only those that you’ll use. Hulu and Netflix allow you to watch two or three screens simultaneously at the same time, which can help you cut costs. And don’t forget to use the cash back app when you shop!
Budgeting
When you are trying to save money for 2022, you have probably thought about creating a budget. Budgeting is an essential tool for financial success and can help you reach your goals, such as a large emergency fund or reducing credit card debt. But how do you make it work? Here are some tips to get you started. Start by setting your financial goals. Then, set aside a certain percentage of your income for each category.
First, remember that winter can be difficult for saving. Comfort foods and heating bills increase your expenses. Saving money is especially challenging during the winter, so start early. The colder weather will make saving easier when the days get longer. Keep these tips in mind and you will have more money in 2022. And remember to make regular savings! Keeping your savings goal realistic can help you reach financial stability. And remember: savings habits don’t have to be complicated. Even if it feels impossible at times, you can always start by examining your current financial situation.
Using energy-efficient appliances
There’s no doubt about it – using energy-efficient appliances can help you save money. They use less energy while performing at a similar level to their comparable counterparts. The manufacturer places an energy label on these products to let you know how efficient they are. This process often works in tandem with Minimum Energy Performance Standards (MEPs), which establish minimum standards for products and their energy efficiency. This can help you save money on your electric bills while at the same time minimizing your carbon footprint.
Many people choose to keep old appliances, which are often expensive to replace. Even though these outdated appliances may save a household money, this expense is often offset by higher utility bills. Using energy-efficient appliances now will help keep a low-income household in its home, as it can lower utility costs. Eventually, those savings can go towards necessities, such as education. Using energy-efficient appliances today can help you save money in 2022 and beyond.
Refinancing
When you refinance your mortgage, you may save a substantial amount of money, but it’s important to keep these costs in mind. The cost of refinancing a home is relatively high, and the savings from a lower interest rate may not start to offset these costs for years. That’s why it’s important to ask yourself four questions before you decide to refinance.
Typically, refinancing will lower monthly payments and save you money over the life of the loan. Refinancing also lets you pull out some equity if you need it, so your payments can go down as well. Depending on your credit history and overall financial health, you may be able to refinance to save money in 2022 and beyond. But refinancing to save money in 2022 can also be a good idea if you are planning to move within the next five years.
Downsizing
There are many benefits to downsizing. One of these benefits is that you can donate the things you no longer use. By donating these items, you can create new memories for others. If you’re thinking about retiring, you can read more on PODS’s blog about where to live and save money in 2022. Here are some tips for downsizing:
Decide what your objectives are before selling your home. Are you looking to be near family, or do you want to be close to shops, restaurants, and transportation? If you’re moving with your family, downsizing your house may not be a good idea. Other people downsize into two smaller houses – one in their hometown, the other in their vacation spot. Regardless of your goals, make sure you research downsizing options thoroughly.

The Best Companies to Work For
While the Fortune 500 ranks the largest corporations in the U.S., the World’s Best list focuses on the people who work at these companies. During a survey, the companies’ employees were asked how they felt about working at those companies. To qualify, a company must […]
BusinessWhile the Fortune 500 ranks the largest corporations in the U.S., the World’s Best list focuses on the people who work at these companies. During a survey, the companies’ employees were asked how they felt about working at those companies. To qualify, a company must be listed on five national Best Workplaces lists and employ 5,000 people globally, with 40% of the workforce located outside of its home country.
Microsoft
One of the biggest reasons why Microsoft is considered the best company in the world to work for is its corporate culture. The company has over 166,000 employees in 177 office locations in 96 countries, and its employee engagement rate is high. Its corporate culture has won several Comparably awards, including Best Company Culture, Best Global Culture, and Best Company Outlook. The company’s biggest focus is empathy, and it encourages employees to learn from real-life experiences. Microsoft encourages teamwork and encourages employees to work across functions.
HubSpot
The world’s most admired companies have a culture that encourages innovation and employee development, and HubSpot is no exception. The company’s remote workforce now consists of more than 300 employees around the world. Remote work practices promote the company’s culture of flexibility. To support remote employees, the company hired a Remote Work and Inclusion Program Manager. The role’s primary goal is to overcome common challenges associated with working from home.
Nvidia is a reputable employer
While it may be easy to believe that all Nvidia jobs are equally good, this is not always the case. In fact, Nvidia’s hiring process can be tough. Nvidia hiring managers are looking for passionate, talented individuals. Nvidia uses artificial intelligence (AI) to review applications and shortlist candidates. In addition, the company is highly selective. To get into Nvidia, you must first be familiar with its mission and culture.
Amazon is controversial
The New York Times recently published an article about Amazon and its culture. Whilst Amazon is a international house-hold name, it is known for creating millions of jobs world-wide. You can always find a job as a delivery driver or working in one of their many large warehouses. That is, those warehouses that haven’t yet been taken over by robot workers! Of course, you can even become a seller and sell your own products on Amazon at a large financial and stressful cost.
Amazon employees described a cutting-edge, intense work environment where employees are encouraged to betray one another in meetings and send each other secret notes. People were also pushed out for voicing personal issues or not meeting company standards. Some employees were so upset by the workplace culture that they cried at their desks. These employees criticized the culture and the company for its lack of respect.
Cisco is a respectful employer
Cisco is a renowned employer with a culture that values diversity, equality and fairness. It prides itself on maintaining a climate of diversity, respect and fairness among its staff. Employees at Cisco are happy to work for the company, which also contributes to various charities and community projects. It has a high employee retention rate with 16.0% of its workforce staying with the company for at least five years. It has a low turnover rate as well, making it a great employer for those looking for a flexible schedule.
Wegmans Food Markets is a multiculteral employer
In a recent study, Zippia rated Wegmans Food Markets a reputable employer. According to the study, Wegmans employees earn a median salary of $30,895. The company has a diverse workforce, with 50% women and 37% ethnic minorities represented. Overall, employees are highly satisfied with Wegmans’ culture and benefits. The company is also a top choice for employees with children, according to the survey.
SAS is a caring employer
The analytics software giant SAS has been named the world’s best employer by the Great Place to Work Institute. It isn’t clear if happier employees are more productive or whether more generous practices increase profitability. Regardless of the answer, SAS has enjoyed 37 straight years of record earnings and a $2.8 billion market cap in 2012. The company’s management practices are unique. They have a long-term commitment to employee satisfaction and provide the opportunity for its employees to grow professionally.

Health Supplements Advice
There are many health supplements available. Many of them are effective, while others have some drawbacks. To get the most out of these supplements, you need to understand the underlying science behind them. To avoid harmful effects, read up on the benefits of each type […]
Beauty & FashionThere are many health supplements available. Many of them are effective, while others have some drawbacks. To get the most out of these supplements, you need to understand the underlying science behind them. To avoid harmful effects, read up on the benefits of each type of supplement. In some cases, you might also need to consult a doctor or healthcare provider before starting a new supplement regimen. But even if your doctor approves, health supplements are generally safe.
Antioxidants include vitamins A, C, and E. These compounds are also present in beta carotene, selenium, and folate. Unfortunately, these have not lived up to their promises. They have not been proven to prevent strokes, cancer, and heart disease. Moreover, they may be harmful in high doses. Omega-3 fatty acids, on the other hand, may protect you from heart disease. However, excessive use of these nutrients may increase your risk of developing heart disease.
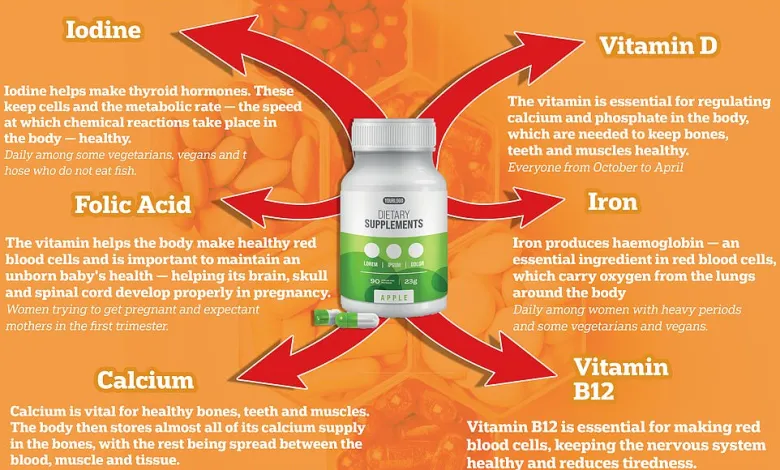
The benefits of health supplements include extra nutrients. They can help you overcome nutrient deficiencies and prevent some diseases. Some health conditions, such as hypertension and spina bifida, can be prevented by taking a health supplement. Taking supplements can help you avoid these medical conditions, and they are often less expensive than medications. But beware of taking too much of any supplement. The risk of side effects is greatest when you take more than you need.
While it is important to take your time in researching the benefits of dietary supplements, there are many risks to be aware of. The benefits of a quality supplement are dependent on your research and common sense. Look for supplements that have high safety standards and are made from quality ingredients. Avoid those that make extravagant claims about their effects. In addition, avoid supplements with a high price tag or are promoted above the recommended dose. You might end up with a nutrient-rich supplement, but you will have to deal with dangerous side effects.
You should consult a doctor before taking any new supplements. Often, they will tell you if you should take them or not. You must follow the directions on the package when taking supplements. Certain supplements can interfere with your current medications. Your health care provider should be able to prescribe a suitable supplement for your condition. However, you should never take more than what is recommended. If you experience an adverse reaction, you should immediately consult your health care provider and report it to the FDA.
While the benefits of taking health supplements are clear, it is vital to read the label carefully. Some can interact with other medicines you are taking, and some may even be harmful. As long as you know what you’re taking and when, supplements can be an excellent tool in your wellness arsenal. However, always make sure to consult your health care provider first before taking any new health supplement. So, what are the risks? Consider all of your options before taking any health supplements.

How to Become a Lawyer
Getting a JD degree can be a good way to start your career. After graduating from law school, you can take up post-graduate studies in the form of a Masters of Laws or Doctor of Laws degree. While a doctorate is not required for practicing […]
Law & LegalGetting a JD degree can be a good way to start your career. After graduating from law school, you can take up post-graduate studies in the form of a Masters of Laws or Doctor of Laws degree. While a doctorate is not required for practicing law, it can increase your knowledge base and expand your job prospects. As of this writing, the process to become a lawyer takes seven years to complete, including four years in undergraduate studies followed by three years in law school.
Law school is a three-year program
The three-year study period of law school can be completed in two to five years, depending on the type of program. In the U.S., the first year of law school is completed during the fourth undergraduate year, and students must meet the requirements for both the General Education and their chosen major. Students must complete 91 credits in their undergraduate degree, of which 29 will count toward the law degree. After four years of law school, students must complete at least seven additional credits to earn their graduate degree.
The second year of law school is often the hardest. Although most first-year classes are graded on a single exam, grades matter a great deal, especially for the prestigious law firms. Most prestigious law firms and judges seek out the top 20 percent of students. The staff of law reviews is also chosen from first-year students. Having good study habits will help you succeed in law school. While the first year of law school may seem overwhelming and demanding, most students say it is worth it. Getting into a good study routine will help you succeed in all three years.
Getting into a good law school
When you’re looking for a good law school, you should make it a point to know the standards of the program. Law schools are looking for individuals who have overcome obstacles, whether personal or professional. Those who have been successful in overcoming challenges bring a diverse perspective to the legal profession. Here are some tips to help you achieve your goals. To get in to a good law school, consider these three tips.
1. Have relevant work experience. Top law schools will look for candidates who can develop arguments and perform library research. Students should have strong analytical skills and an excellent LSAT score. In addition, they should be proficient in language. If you are a non-native English speaker, you should obtain a relevant language qualification. The deadline for applying to law school varies, so be patient. Once you’ve gotten an acceptance letter, you can focus on writing the essay or resume that shows how you’ve used these skills.
Passing the bar exam
After earning a law degree, you will be required to pass the bar exam in your state of choice. Each jurisdiction has its own bar exam requirements, although some may have reciprocity. The exam is typically held twice a year, in July and February, with results published about three to four months later. To prepare for the exam, consider reading through NCBE-approved bar review materials. Practicing in a law office is an excellent way to gain experience and learn more about the legal field.
The MPT consists of two 90-minute problems covering state-specific law. Examiners must identify relevant facts, evaluate inconsistencies in source documents, and use the resources in the Library to complete a particular lawyering task. This might include preparing a settlement agreement, drafting a brief, preparing a discovery plan, or composing a letter to a client. It is essential to study thoroughly and allocate time effectively between each section.
Working as a lawyer
Becoming a lawyer is not for the faint of heart. Although the pay can be lucrative, working as a lawyer requires long hours of study and preparation for each case. Not only will you be exposed to high-stakes scenarios, but you will also be required to take care of clients. Even if your first assignments are low-stakes, you will quickly find yourself working on high-stakes cases.
If you’re considering leaving the profession, it’s a good idea to write down what you hate about the profession. Many disgruntled lawyers can articulate a long list of complaints, but they can also articulate ways of working within the limits of the profession. Working as a lawyer is a demanding job, but it also offers the chance to learn and experience the nuances of the profession.

Can I Get Legal Aid For Free?
Obtaining legal representation is often the best course of action for those facing disputed issues in court. But what happens if a defendant cannot afford to pay for legal counsel? Is free legal aid available? To know how I can get Legal Aid For Free? […]
Law & LegalObtaining legal representation is often the best course of action for those facing disputed issues in court.
But what happens if a defendant cannot afford to pay for legal counsel? Is free legal aid available?
To know how I can get Legal Aid For Free? Schedule a free initial consultation with an experienced lawyer.
This blog post will explore the pros and cons of getting free legal aid to help you decide whether it’s an option worth considering.
What is Legal Aid?
Legal aid is any form of assistance that is provided to people who are unable to afford legal representation.
This can include advice on various cases (including criminal ones), representation during court hearings, and financial assistance for lawyer’s fees.
Who Qualifies for Free Legal Aid?
Qualifying criteria for free legal aid differs between jurisdictions. Still, generally speaking, those who meet certain income and asset requirements may be eligible depending on the severity/complexity of their case and other factors such as special circumstances surrounding them (i.e. disabled individuals etc.).
Furthermore, some states may offer pro bono services, too, meaning lawyers working on behalf of those who can’t afford it.
Pros Of Obtaining Free Legal Aid
The most obvious advantage of obtaining free legal aid is cost savings since professional fees can vary greatly depending on the nature and complexity of one’s case.
Moreover, those legally represented through such programs may also benefit from more experienced professionals (albeit limited) compared to self-representation and access to high-quality research tools/resources needed when preparing defenses against charges levied against them during trials.
Cons Of Obtaining Free Legal Aid
Unfortunately, there are several downsides associated with getting free legal aid – one being restrictions imposed upon services offered by pro bono lawyers (due to pre-set budgets assigned etc.).
Additionally, it can take some time before one finds an adequate lawyer willing to work within set limitations; they may need more time to complete paperwork or court proceedings before deadlines set forth by relevant authorities leading to potentially higher costs overall!
Advice For Those Seeking Free Legal Aid
If you’re considering getting free legal aid, ensure that you seek out local resources first while being aware of potential restrictions imposed upon them, such as limitations upon monetary compensation offered.
Additionally, look out for organizations offering discounted/pro bono services depending upon the type/severity of your case; doing so usually helps narrow down search times and allows individuals more control when choosing who represents them during their trial.
Conclusion
Free legal aid may be challenging for some but rewarding in the long run – ensuring that individuals understand the various stipulations involved beforehand is imperative so that they can make an informed decision regarding whether such options are suitable for their current situation.
Remember that seeking out reliable organizations should always come first over relying solely on government/state-funded programs – consulting with attorneys about different plans may also prove useful before starting any paperwork today.

by Randal Spain
How to Choose the Right Garage Door for Your Home
Your garage door is a huge part of your home. It not only keeps your car safe, but it also plays a role in the overall look and feel of your property. If you are looking to replace your old garage door or install a […]
Home ImprovementYour garage door is a huge part of your home. It not only keeps your car safe, but it also plays a role in the overall look and feel of your property. If you are looking to replace your old garage door or install a new one, it is important to choose the right one for your home. In this blog post, we will discuss the different types of garage doors available and help you decide which one is right for you!
Choosing a Material
One of the first things you need to consider when choosing a garage door is the material. Garage doors are typically made from wood, steel, aluminum, or fiberglass. Each material has its own benefits and drawbacks that you need to consider before making a decision, however, if you are still unsure about the right material for your garage door, your local humilton garage door installation company will be happy to help.
Wood
Wooden garage doors are classic and elegant. They can be painted or stained to match the exterior of your home. However, wood is also susceptible to rot, insects, and weather damage. If you choose a wooden garage door, be sure to inspect it regularly and perform any necessary maintenance.
Steel
Steel garage doors are strong and durable. They are low maintenance and resistant to weather damage. However, steel doors can dent and Rust over time. If you choose a steel door, be sure to inspect it regularly for any signs of damage.
Aluminum
Aluminum garage doors are lightweight and low maintenance. They are also resistant to weather damage. However, aluminum doors can dent and scratch easily. If you choose an aluminum door, be sure to inspect it regularly for any signs of damage.
Fibreglass
Fibreglass garage doors are strong and durable. They are also low maintenance and resistant to weather damage. However, fibreglass doors can break easily. If you choose a fibreglass door, be sure to inspect it regularly for any signs of damage.
Types of Garage Doors
Once you have decided on a material, you need to decide what type of garage door is right for your home. There are three main types of garage doors: roller, sectional, and side hinged.
Roller Garage Doors
Roller garage doors are the most common type of garage door. They roll up and down to open and close. Roller garage doors are easy to use and require little maintenance.
Sectional Garage Doors
Sectional garage doors are made of sections that hinge open and closed. Sectional garage doors are more expensive than roller garage doors, but they offer better insulation and security.
Side-Hinged Garage Doors
Side-hinged garage doors open like a door on hinges. Side-hinged garage doors are easier to repair than other types of garage doors, but they can be difficult to open in windy conditions.
Choosing a Colour
The nice thing about garage doors is that you can get them in any colour you want. You can match the garage door to the trim on your house or go for a contrasting look. If you are unsure what colour to choose, we recommend consulting with a professional or visiting a garage door showroom.
Final Thoughts
Choosing the right garage door for your home can be a difficult decision. There are many factors to consider including material, type, and colour. Hopefully, this blog post has helped you narrow down your choices and made the decision-making process a little easier. Happy garage door shopping!

by Randal Spain
Misconceptions Surrounding Criminal Defence Lawyers – Debunked!
Movies and TV shows can often depict criminal defence lawyers as scheming individuals accumulating money by lying in court for a living. As common as this misconception may be to the general public, it is far from an accurate depiction of a real-life criminal justice […]
Law & LegalMovies and TV shows can often depict criminal defence lawyers as scheming individuals accumulating money by lying in court for a living.
As common as this misconception may be to the general public, it is far from an accurate depiction of a real-life criminal justice attorney in Toronto. Here are a few common misconceptions people hold about criminal lawyers that just aren’t true-
3 Misconceptions Surrounding Criminal Defence Lawyers
1. Any Qualified Lawyer Can Defend In A Criminal Case
Lawyers specialize in certain fields to provide the best results to clients through their expertise and knowledge. If a domestic case like divorce comes up, it will make no sense to hire an entertainment lawyer to get a better outcome.
Similarly, you need someone who is well-versed in criminal law so that they can assist you correctly. A good lawyer can present your case and articulate your rights effectively, which might even lead to reduced sentences or fines.
2. Public Defenders Are As Good As Criminal Defence Attorneys
The thing is, public defenders are overworked and underpaid individuals who are swimming knee-deep in a pile of cases. With the number of ongoing cases in their hands, it is not possible for them to dedicate time, effort, and attention to individual cases.
Personalized focus on the case is lost, and you may not get the opportunity to communicate properly within the limited time allotted to your case. The second issue is — there are a handful of good public defenders with the right skills to manage your case, but most of them are inexperienced.
Public defender offices are full of fresh-out-of-school attorneys who do have enough knowledge or experience to tackle criminal cases. This is why it is better to hire a criminal defence lawyer instead of relying on public defenders.
3. Criminal Defense Lawyers Have Questionable Morals
Popular media likes to show criminal defence lawyers as shady individuals that help criminals get away with crime. While this trope makes for entertaining movies, it is not entirely in touch with reality.
Of course, there are some underhanded individuals in every profession; however, most criminal defence attorneys have a stable moral compass. They have a sense of understanding that they need to balance both — ethics and service to a client.
Lawyers spend years in the training process and invest huge amounts in going to law school. It is very unlikely that they will be willing to throw all that away only to indulge in wrongdoings.
Final Words
As someone who’s never needed to step into court before, you may not be fully aware of court procedures and the importance of criminal defence lawyers. Thus, it is possible to have misconceptions like assuming that criminal defence attorney consultations are free.
However, it’s crucial to get in touch with the right lawyer for a criminal case. After all, you don’t want to put your future and life in the hands of an inexperienced lawyer or one who takes criminal cases occasionally.

by Randal Spain
4 Reasons to Arrange a Legal Consultation After a Motorist Offence
No one ever expects to be pulled over by the police and given a ticket for a traffic offence. But it happens every day, to millions of people. If you find yourself in this situation, don’t panic. There are things you can do to minimize […]
Law & LegalNo one ever expects to be pulled over by the police and given a ticket for a traffic offence. But it happens every day, to millions of people. If you find yourself in this situation, don’t panic. There are things you can do to minimize the damage, including arranging a legal consultation. In this blog post, we will discuss four reasons why you should seek legal advice after being issued a motorist offence.
Reason 1: To Understand the Severity of the Offence
The first reason you should get legal consultation in London is to ensure you understand the severity of the offence. Depending on the traffic offence, you could be facing a minor fine or a major penalty. In some cases, you may even lose your licence. A lawyer will be able to explain the consequences of your actions and help you determine the best course of action.
It is vital the drivers understand the severity of their actions and the possible punishments before taking any further steps. Otherwise, you could be inadvertently making the situation worse.
Reason 2: To Learn About Your Rights
You may feel like you are out of options when you are pulled over and issued a ticket. But this is not the case. You have rights as a motorist, and it is important that you are aware of them. A lawyer will be able to advise you of your rights and help you understand how to protect them.
For instance, many people do not realize that they have the right to contest a traffic ticket. If you believe that you were unfairly ticketed, you can fight the charges in court. But this is not something you should do without legal guidance.
Reason 3: To Get the Best Possible Outcome
The goal of any motorist who is facing a traffic offence is to get the best possible outcome. This may mean paying a small fine or avoiding a licence suspension. But it could also mean getting the charges dropped entirely.
A lawyer will be able to assess your case and advise you of the best way to proceed. They will also be able to negotiate on your behalf with the prosecutor. In many cases, this can result in a more favourable outcome than if you had attempted to deal with the situation on your own.
Reason 4: To Avoid Future Offences
If you are convicted of a traffic offence, it will go on your permanent record. This can make it difficult to get insurance or even rent a car. And if you are convicted of multiple offences, you could lose your licence altogether.
A lawyer can help you avoid a conviction by negotiating a plea deal with the prosecutor. In some cases, you may be able to get the charges dropped entirely. But even if a conviction is inevitable, a lawyer may be able to help you get a reduced sentence.
Final Thoughts
Dealing with a traffic offence can be a stressful and confusing experience. However, if you are in this position, it is important to remember that you are not alone and that having a lawyer on your side can make a big difference.
If you have been charged with a traffic offence, be sure to schedule a consultation with a lawyer as soon as possible. They will be able to advise you of your rights, help you understand the severity of the offence, and work to get the best possible outcome for your case alongside many other benefits.

by Randal Spain
5 Tips for Fighting Drug Charges: How to Avoid a Conviction
If you have been charged with a drug crime, you are probably feeling scared and overwhelmed. You may be wondering how you can possibly fight the charges against you and avoid a conviction. The good news is that there are several things you can do […]
Law & LegalIf you have been charged with a drug crime, you are probably feeling scared and overwhelmed. You may be wondering how you can possibly fight the charges against you and avoid a conviction. The good news is that there are several things you can do to improve your chances of success. In this blog post, we will discuss five tips for fighting drug charges.
Tip 1: Hire an Experienced Criminal Defense Lawyer
If you have been charged with a drug crime, the first and best thing you can do is to hire an experienced criminal defense lawyer. With so many criminal lawyers in Scarborough, finding the right one can be difficult. However, it is worth the effort to find an attorney who has experience handling drug cases in your jurisdiction and who you feel comfortable working with.
Tip 2: Get Help From a Drug Rehabilitation Program
If you are struggling with addiction, getting help from a drug rehabilitation program can be an important step in fighting your drug charges. Many courts are willing to be lenient with defendants who can demonstrate that they are taking steps to address their addiction. Similarly, prosecutors may be more likely to offer a plea deal if they believe that the defendant is serious about getting treatment.
Tip 3: Challenge the Police’s Search and Seizure
In many drug cases, the police have obtained evidence through illegal search and seizure. If this is the case in your case, your attorney may be able to get the evidence against you suppressed. This can be a complicated legal issue, so it is important to have an experienced criminal defense lawyer on your side. They will know exactly where to look for evidence of illegal search and seizure and how to challenge it in court.
Tip 4: Evaluate Your Options for a Plea Deal
If the evidence against you is strong, you may want to consider pleading guilty in exchange for a lighter sentence. This is known as a plea deal. Plea deals can be beneficial because they allow you to avoid the risk of a more severe sentence if you are convicted at trial. However, they also have some drawbacks. For example, pleading guilty will result in a criminal record. You should discuss all of your options with your lawyer before making a decision.
Tip 5: Use an Expert Witness
If you have been charged with a drug crime, you may be able to use an expert witness to your advantage. An expert witness is a person who has specialized knowledge about a particular topic. They can provide testimony that can help to disprove the prosecution’s case or cast doubt on their evidence. This can be an effective strategy, but it is important to make sure that you find an expert witness who is credible and who will be able to present their testimony in a clear and convincing way.
Final Thoughts
The effects of drug charges can be serious and long-lasting. If you have been charged with a drug crime, it is important to take the time to understand your options and make the best decisions for your case. With the help of an experienced criminal defense lawyer, you can give yourself the best chance of success so be sure to contact one if you find yourself in this situation.

by Randal Spain
5 Ways to Look Your Best Without Surgery
Everyone wants to look their best. If you take a lot of pride in your appearance and want to keep your healthy and youthful look, you may have been tempted to get cosmetic surgery. Surgeries are very expensive and have a lot of side effects, […]
Beauty & FashionEveryone wants to look their best. If you take a lot of pride in your appearance and want to keep your healthy and youthful look, you may have been tempted to get cosmetic surgery. Surgeries are very expensive and have a lot of side effects, so they generally should not be the first treatment option. Luckily, there are many ways to look your best without surgery. This article will examine five alternatives you can try today.
1.) SBLA Wands
SBLA wands offer many of the benefits of facial surgery without the cost and nasty side effects. They offer a full collection of serums in wand applicators that each improve the appearance of skin. The SBLA face wand features concentrated ingredients in a time release formula that increase the skin’s moisture, decrease the appearance of lines and wrinkles, and tighten skin to keep it looking young.
2.) Microdermabrasion
Microdermabrasion is an alternative to surgery that must be completed in a skin care professional’s office and requires a local numbing agent. There are several types of microdermabrasion that each involve slightly different equipment and procedures. They all work by exfoliating the skin to remove the thick outer layer and include benefits such as treating acne and reducing the appearance of age spots and wrinkles.
3.) Topical Olive Oil
Olive oil is great for skin. It increases moisture and contains loads of antioxidants. Olive oil can be found in many skin care products, but you can also use the oil you probably already have at home for cooking. If you have dry skin, apply a very thin layer of olive oil to your face and leave it on for a couple hours or even overnight. It will protect against environmental damage, water loss, and premature aging.
4.) Diet and Exercise
Exercise and a healthy diet are not glamorous, but their importance in looking great cannot be overstated. Weight control is an obvious benefit but there are many others. High antioxidant foods protect skin against damage from the sun and other environmental toxins. Vitamin-rich foods aid skin healing to keep it looking clear and healthy. Exercise increases blood flow to your skin, which keeps it glowing and youthful.
5.) Get Adequate Sleep
Sleep does some amazing things for the body. You probably already know that getting good sleep makes you feel great, but it can also keep you looking great. Most people don’t get enough sleep and it shows. You produce collagen during sleep which keeps skin smooth and supple. Getting plenty of rest also prevents puffy eyes and dark undereye circles. Don’t underestimate the importance of sleep in your beauty routine.
Final Thoughts
Cosmetic surgery can be tempting, with its claims of quick results and little effort. However, the associated cost, pain, side effects, and complications make surgery a poor first choice to improve your appearance. Prioritize healthy habits and take advantage of some of today’s amazing non-invasive technology, such as SBLA wands and microdermabrasion to get many of the benefits of surgery without the side effects.

by Randal Spain
What Type Of Breakfast Can You Enjoy In A Popular Restaurant?
Breakfast is the first meal of the day after a long sleep at night; thus, it should be healthy and full of nutrients. In addition, breakfast enhances energy levels and helps to work very fast in the morning by improving the ability to concentrate. Moreover, […]
FoodBreakfast is the first meal of the day after a long sleep at night; thus, it should be healthy and full of nutrients. In addition, breakfast enhances energy levels and helps to work very fast in the morning by improving the ability to concentrate.
Moreover, one should never skip this meal as it reduces the risk of several heart diseases and diabetes. Also, eating breakfast assists in controlling the weight, which diminishes other serious problems.
In contrast, breakfast is the initial thing or food that is served in a restaurant or a hotel. These places have amazing foodstuff that can provide an excellent start to the day. Also, a few restaurants only offer breakfast, or some provide brunch (lunch and breakfast together).
Continental Breakfast
It is one of the most prevalent meals preferred by people all over the world. Continental food is easy to digest by the body because it is very light. In addition, these breakfasts are designed in a way so that they can easily be stored anywhere.
The continental breakfast is usually placed in the buffets where people serve food by themselves. This type of foodstuff is usually found and eaten in European countries, and the name of the breakfast was first introduced in the year 1896. Also, you can have wonderful continental food in breakfast restaurants Toronto.
The menu of the continental breakfast consists of different items such as:
- Fresh Fruit Juice
- Assorted Bread (croissants, muffins, etc.)
- Beverages(coffee/tea)
The continental breakfast is served in a classic manner, and everything is placed in a decided position. The items utilized in the breakfast are:
- Napkin: A tablecloth or napkin is put under the side plate and knife.
- Plate and Knife: In the middle of the table, a knife is placed on the right of the side plate, putting the cutting side on the left.
- Cup and Saucer: The cup is sited on the right-hand side at a 120-degree angle, making it convenient to carry, and a spoon is put on the saucer.
American Breakfast
This type of breakfast is quite different from the continental one as it is neither too heavy nor too light. The most common drink desired by many customers is coffee.
Usually, cold water is provided before serving any dish in various restaurants. American breakfast doesn’t offer fish in the course like the English breakfasts. Instead, the menu consists of cereals, eggs, pancakes, and fruits.
English Breakfast
It is one of the heaviest early meals, which are mostly served in the country of the UK, Ireland, etc. The name of the breakfast can be different in the respective regions in which it is eaten. People looking for low-calorie and high-fiber foodstuff can go for this, as it includes low cholesterol butter, boiled vegetables, skimmed milk, etc.
FAQs
- What is considered a healthy breakfast?
Ans.: Breakfast that doesn’t have unprocessed food and includes vegetables, grains, and fruits are said to be a healthy meal.
- What are the perks of eating Breakfast regularly?
Ans.: One should eat breakfast daily because it increases energy level, helps lift mood, boosts the immune system, etc.
- What is the best time to eat Breakfast?
Ans.: The primary time for eating early meals is between 6 to 10 am.
Therefore, you can get several food items for your morning meals in various restaurants. These meals are healthy and help in many physical activities throughout the day.

by Randal Spain
How to Create Unique and Awesome Looking Gift Baskets?
Do you want to give someone a gift basket? You should give them a gift basket! However, most people just purchase the special gift basket, but creating a unique one will be thoughtful. If you think it is not easy to create, then you are […]
ShoppingDo you want to give someone a gift basket? You should give them a gift basket! However, most people just purchase the special gift basket, but creating a unique one will be thoughtful. If you think it is not easy to create, then you are wrong! Because it is easy to assemble as you can personalize it with the things you want to put in.
In fact, these gift baskets toronto, Ontario, are suitable for every occasion and are the solution if you cannot decide what you should give to the person. The basket size will depend on you and also decide what you want to put in it. If you want to create the unique one, then here are some things you can consider.
Choose the theme
At first, you should select the theme, which will be possible when you focus on the individual’s interest. From there, you can select the suitable theme, and then only you should select the available items. For instance-
- For a gardener, you can consider getting the basket with the seed packets and tools for gardening.
- For someone who has just bought a house, you can give them something such as essential oil, scented candles, or other small tools they may need.
It does not matter what you are giving; make sure the other will love it if you give them with love and a smile.
How can you assemble the gift basket?
Assembling the gift basket is one of the essential tasks, and most people cannot do that. Look below to know how you can do that-
- First, place the paper doily or cloth napkin at the bottom and then arrange the entire item inside it. Make sure if there are any breakable items, then they should be wrapped properly. You can even wrap some candy on the items to give a nice touch.
- Cut the two yards of cellophane- special occasion printed and then place it on the surface of the table upside down. Then put the basket in the middle.
- Gather the cellophane, pull it up, and then secure it with a long twist.
- You can even add a ribbon to it, giving it a nice touch. You can also add the gift tag on top of it.
Some essential tips you should know
- Using ready-made bows is best, and you will not have to face any problems creating one. But ensure you are getting the one that fits your basket perfectly.
- Several other things can also be used instead of a basket, such as a pie plate, serving bowl, gift box bottom, and many more. You can choose the one that will make it easy for you to make a better thing.
Conclusion
Creating the gift basket is the best choice for the person, as it tells the other person that you love them the most. But you must ensure you are giving the best gift they will need and use.

by Randal Spain
10 Basic And Interesting Aspects Regarding Criminal Law Jobs
Do you have to respond to any criminal charges? Several people do have to respond to them as they have to appear in court and deal with enforcement, court staff and prosecutors. Many people who have not been convicted of any crime may also have […]
Law & LegalDo you have to respond to any criminal charges? Several people do have to respond to them as they have to appear in court and deal with enforcement, court staff and prosecutors. Many people who have not been convicted of any crime may also have to undergo several procedures. It is impossible to handle these things, which is why they require legal consultation in winnipeg.
Getting the best lawyer to give you the best legal advice is essential to winning the case and making things better for you. Of course, nobody wants to go to court again and again. But, with the help of these criminal lawyers, that can be possible.
What is criminal law?
Criminal law is basically the law that is related to crimes. It is a system of laws that will be concerned with the punishment of those people who have committed any crimes. A criminal prosecution does involve the government deciding whether the person needs to be punished or not.
Interesting aspects regarding criminal law jobs
- Criminal lawyers are quite serious about their work, people who have been charged with the crime will need some help, and they will be the ones who can provide them. Therefore, how they feel personally about the crime will not affect the representation.
- Every criminal prosecution has the right to have a criminal defence lawyer who will represent them in court.
- You may have seen several cases settled before the trial, which is possible if your lawyer has researched the jury. When they research about them, then that will help you reach the final verdict.
- A criminal lawyer can watch the jury’s body language, which will help them make the decision. They will know whether the other person is lying or telling the truth.
- The criminal lawyer always supports their clients no matter what happens, and they even ask their clients not to talk about their cases anywhere. However, if they do so, that may lead to several problems and negative consequences.
- If you are in a criminal law job, they usually love to take those cases they cannot win. They want to challenge their skills, experience and training that will help someone who needs help.
- Criminal defence lawyers know that Jurors watch the police show and true crimes, and in some cases, jurors think they know everything. And a criminal lawyer will know how they need to prepare the case.
- Jurors are also involved in many cases and influenced by the news, which is spread by word o mouth. Lawyers may know this and that, so they keep everything in mind and work on the defence.
- If you choose the best criminal defence lawyer, you will be sure that you cannot tell anyone about the client’s confession. They cannot reveal anything about the client.
- A criminal lawyer will even know how they can fight against the forensic evidence and even challenges that too.
Conclusion
Criminal lawyers play an essential role in the life of people who have committed a crime or were convicted of one. But make sure you are choosing the best lawyer who has all the qualities and experience.

5 benefits of mental health counselling
Life is full of uncertainties, and sometimes, things can get rough. In such times, we may need someone to help us get through the difficult period. Whether online or offline, counsellors are easily accessible in Canada. If you are dealing with grief, depression, anxiety, relationship […]
FitnessLife is full of uncertainties, and sometimes, things can get rough. In such times, we may need someone to help us get through the difficult period. Whether online or offline, counsellors are easily accessible in Canada. If you are dealing with grief, depression, anxiety, relationship issues, etc., you can contact a counsellor in St. Catherines or near you. They can help in many ways. Whether you are living with a mental illness or not, your mental health can benefit greatly from counselling. This blog shares five ways in which counselling improves our mental well being.

1. Improves self-esteem and self-acceptance
You may experience things that cause you to feel insecure, not just in physical appearance but in your ability or personality. We all have flaws, and no one is perfect. But some may go through things that cause them to dwell too much on their imperfections. In times like that, with a counsellor’s help, you can learn to accept yourself the way you are. You can learn to be compassionate with yourself, and this will raise your self-confidence.
2. Helps you look at problems from a different perspective
People think of mental illnesses when they hear the word counselling or therapy. However, those without mental health issues are not exempted from life’s problems. Once in a while, life can throw problems at you that leave you confused or simply stressed. Counselling can help you deal with life’s issues by helping you see problems in a different light.
3. Increases your self-awareness
Counselling gives you the opportunity to learn more about yourself and your emotions. Talking to a counsellor helps you develop valuable insight into your personality, beliefs, and values. This increases your self-awareness and enables you to change and grow as a person.
4. Helps you deal with mental health issues
Counselling can benefit you immensely if you are struggling with a mental issue, such as depression, anxiety, etc. You will learn about the mental health condition, including the source of the problem. You will also be exposed to the different treatment options to help eradicate the symptoms. Counselling sessions will help you learn different ways to cope with the condition. This will greatly improve your mental well-being and soon lead to full recovery.
5. Provides a safe place to discuss your feelings
Counselling offers a safe place for anyone to talk about their issues without fear of judgment. Professional counsellors must possess these three important traits:
- Understanding
- Empathy
- Non-judgemental
That is why you will feel like your situation is normal in a counselling session. The counsellor provides a supportive environment that makes you feel validated and safe.
Conclusion
Counselling offers many benefits, and the few listed here shows that anyone can benefit from it. If you are already dealing with a mental illness, counselling sets you on the path to recovery. If you are fortunate enough to have no mental health issues, it is unlikely that you have lived with zero challenges till now. As life’s issues arise, counselling helps you deal with the emotions these issues generate. Through this, your mental health stays intact and continues to improve.

by Randal Spain
Long Distance Air Travel Vs. Ground Transport: What’s Best for My Puppy?
Are you about to move and pet relocation is on your mind? More specifically, are you conflicted between air travel and ground transport? Well, fret no more. We’ll assess the pros and cons of both and determine which method will be best to relocate your […]
Pets CareAre you about to move and pet relocation is on your mind? More specifically, are you conflicted between air travel and ground transport? Well, fret no more. We’ll assess the pros and cons of both and determine which method will be best to relocate your puppy.
The following pros and cons apply to both – relocating your puppy yourself or hiring a pet relocation service to do it for you.
Relocating your puppy by air travel
Air travel is definitely faster. If time is a concern, then there’s really no doubt here that you should go with air. But at the same time, it’s also generally more expensive unless no extra commute is required.
Some dogs might not like to travel a lot and in their case, air travel becomes the only option. Shipping a small puppy is not very expensive as compared to large dogs or crates of pets – so don’t decide on anything by looking at the average pet relocation costs for air travel. Those are mainly for larger pets.
One of the disadvantages is the drive to and from the airport. There are also many limitations in animal airline travel – so you better check for the breed, size, number, crate size, etc.
Aircraft type and cargo facilities also affect your options.
If you’re sending your puppy by plane then know that it won’t be supervised. Pets are put in the cargo area with no humans. Health checkups before and after the flight are critical in the case of air travel. The process of booking, checking in, etc. is also different and can become complicated.
Lastly, flights are prone to delays and cancelations and then there’s the issue of the weather. This can all cause additional stress both for your pet and you.
Relocating your puppy by ground transport
Ground transport is better than air travel for any puppy because you can have supervision and care throughout the journey. It’s longer but also less expensive and more hassle-free.
Ground travel is recommended when safety and health are concerns. For example, not all puppies will adapt well to a cargo shipping experience. Ground travel, on the other hand, ensures activity.
You might need to pay for the handler – but that will additionally ensure you get pictures, food, toys, the whole thing.
There are also generally fewer or no restrictions when transporting pets by ground. If taking them with you is not an option, the ground is obviously the safer option of the two.
Going longer distances on roads can actually become more expensive too – especially because all ground-based pet relocation services charge on mileage.
Final verdict: Air or ground?
Generally, ground transport is considered to be better for small puppies. However, there’s a lot to factor in. In many cases, air travel can actually be a better option for your canine.
Review all your options carefully. Ultimately, only the pet owner can make the best decision for their pet.
If you are looking for Toronto dog shipping, then look no further than Paws en route. We provide the best-in-class pet relocation service, both air and ground, with great care in Toronto, Montreal, Calgary, and Vancouver.

Should I Hire an in-field Marketing Agency?
No matter the services, products, or size of a business, marketing is indispensable for every company. The goal of every business is revenue. And revenue can only result from: Marketing is all about creating awareness and getting customers. Companies have two ways of taking care […]
BusinessNo matter the services, products, or size of a business, marketing is indispensable for every company. The goal of every business is revenue. And revenue can only result from:
- Creating awareness for the products or services
- Getting customers to buy
Marketing is all about creating awareness and getting customers. Companies have two ways of taking care of this:
- Hiring marketing staff and creating an in-house team
- Outsourcing the work to a marketing agency.
Which method would you choose if you need a marketing team to go out in the field to promote your brand and generate leads? Many Canadian businesses seeking in-field marketers are now choosing the latter. But before you decide, consider these points while comparing both ways:
1. Time
Hiring or expanding your own in-house team will require:
- Attracting applicants
- Selecting potentially suitable candidates
- Conducting several interviews
- Training
This process can take several months to develop an effective in-field marketing team. Hiring staff alone, apart from training, will require at least one month.
In contrast, outsourcing to an agency requires:
- Setting up interviews/meetings with agencies (within one week)
- Choosing a suitable agency and signing contracts (2 – 3 days)
- Field marketing begins (within two days)
So if you wish to have a team ready within two weeks or less, an agency is the best bet. It is possible to hire a competent in-field marketing agency in Toronto or near you sooner than you expect.
2. Cost
In-house team costs include the following:
- Recruitment costs
- Training
- Replacement costs if a member leaves the team
- Employee benefits costs
A marketing manager’s average annual salary can reach $120000. Now add average annual pay for other members of the team.
Contrary to this, the average monthly retainer for a marketing agency in Canada ranges from $2500 to $9000. The agency handles the team, and there are no other expenses, such as employee benefits.
3. Control and communication
The in-house marketing team allows you to monitor your staff’s work closely. The proximity lets you ensure the team complies with the company’s objectives and vision. An in-house field marketing team is sure to adhere to the company’s work culture and code of ethics.
On the other hand, it is tough to monitor an external team’s activities. You will have to rely on communication, which might not be as fast as dealing with an in-house team. The agency may also need time to adapt to the company’s culture. However, this could bolster their creativity and innovation.
Both choices offer different levels of communication and control. Communication can continue to improve with time. But if you prefer a higher level of control, an in-house team might be best.
Final thought: should I hire an in-field marketing agency?
That depends on your goals and objectives. Consider the points we explained in this blog to determine what’s best for your business. If time and cost are major factors, then hiring an agency is the better option for your business. But if you seek more control of your team, consider hiring or expanding your internal staff.

by Randal Spain
4 Signs of a Septic System Failure
People’s homes are connected to the public sewer system. But they’ll probably need a septic tank if they live in a rural area. Septic tanks are very helpful for homes and the community as a whole. Septic tanks are made to store household wastewater underground […]
Home ImprovementPeople’s homes are connected to the public sewer system. But they’ll probably need a septic tank if they live in a rural area.
Septic tanks are very helpful for homes and the community as a whole. Septic tanks are made to store household wastewater underground so that it can be treated easily. Septic tanks run domestic sewage systems, usually made of plastic, fiberglass, and concrete.
When septic systems fail, sewage may overflow and rise from the ground. If you are connected to a septic system, it will be your job to keep it in good shape. Even if maintaining your septic system doesn’t seem like a hard job, it may take a lot of work. Choosing Bethany septic tank installation is the best thing to do, if you want to make things easy for yourself.
SIGNS OF A SEPTIC SYSTEM FAILURE
This article provides four tell-tale signs you should look for in case of system failure.
- SEPTIC SYSTEM BACKUP
- NASTY ODORS
- GREEN GRASS
- SLOW DRAINS
SEPTIC SYSTEM BACKUP
Every plumbing fixture in your house is connected to the septic system. When your septic tank is broken or full, wastewater and sewage won’t go in. It will stay there until the water begins to rise.
Because of this, sewage and other wastewater flow back into your toilet, sinks, and drains. This is the clear sign of some problem in the septic tank which is getting worse. If you see this sign, you should immediately call a professional plumber.
NASTY ODORS
Bad smells in the vicinity of the drain field and plumbing indicate that a septic system is failing. Your septic tank may have failed if you can smell strong, unpleasant scents when you go outdoors and onto your lawn.
You might reach a similar conclusion if the same offensive scents permeate your house. These foul smells indicate sewage is present because it has entered your main drain line after cresting the drain field.
GREEN GRASS
While this could be beautiful to see, it might also signify something more serious. The grass above the sewage tank may normally be quite dry. This makes sense that the soil underneath would be deeper than the surrounding region. It should be more luscious.
But it means too many nutrients and water are being dumped into the drain. Your grass will get fertilized by the wastewater, which will cause it to grow tall and lush. Even if it sounds good, it might be bad for your health.
SLOW DRAINS
A broken septic tank could also cause slow drainage. For instance, a full septic tank will not actively collect wastewater. This means that the sewage in your pipes will get clogged, making it hard for them to drain your plumbing fixtures.
Your drains start to slowly release water or other liquids on their own. You won’t be able to clear your drain with any tool because the problem is in the septic tank.
Slow drains are a sign of a full septic system backlog, which makes your drains completely un-operational and causes wastewater to back into your house.
CONCLUSION
After reading this article, you ought to have a better grasp of the signs that the septic system in your house isn’t working. You should be aware of how well your septic system is working and when it requires emptying.

by Randal Spain
Before you choose a chiropractor: 5 things to keep in mind
Choosing a chiropractor to treat your back pain is a big decision. You want to make sure that you find a good one who will help you recover and relieve your pain. The first thing to keep in mind is that there are many different […]
AutoChoosing a chiropractor to treat your back pain is a big decision. You want to make sure that you find a good one who will help you recover and relieve your pain.
The first thing to keep in mind is that there are many different types of chiropractors. The type of chiropractor you choose will depend on your symptoms, the area you live in, and the costs of treatment. Here are 5 things to keep in mind before you choose a chiropractor:
1. Do they offer the right treatment?
You should ask yourself if they offer the right treatment for your condition. You can ask directly to find out if they offer the right treatments. Check out at their website and see what types of treatments they offer so you have an idea of whether or not they know what they are doing.
2. Are they experienced?
You want to make sure that the chiropractor has experience treating back pain and other spine-related issues like sciatica, headaches, and migraines because this will help them treat your condition much better than someone who hasn’t dealt with these issues before.
The best way to do this is by asking questions about their experience so that you know how proficient they are at treating this type of problem before hiring them as your chiropractor.
More experpeiced chiropractors can also troubleshoot other underlying medical issues.
3. Do they have a license?
Find someone who is licensed by your state or province.
You want to make sure that the person has been certified by their state’s board of chiropractic, and that they have been practicing for at least three years.
4. Do they offer insurance reimbursement for treatment plans?
Many clinics offer partial or full reimbursement for treatments, which can save you money if your insurance plan doesn’t cover all costs of treatment.
This can also be helpful if your insurance plan requires pre-approval before receiving services from a particular provider, but it is more difficult to find out about this information from the clinic itself due to privacy concerns.
5. Ask about their past customers
Ask about their knowledge base and experience with each patient’s unique needs and concerns about pain relief and wellness.
You want someone who knows how to address specific issues that may arise after treatment, such as what exercises might help control back pain or how long it takes for the treatment.
In conclusion
Choosing the right chiropractor is important for your health. If you’re new to the world of chiropractic care, it can be hard to know where to start.
If you want to find a good chiropractor that will help you get better, then we recommend the leading physitherapy oakville: We-Fix-U. With a proven track record and overwhelmingly positive patient reviews, they are the right fit for anyone looking for a professional physitherapist

4 Steps to Sending Flowers to Someone in Brampton
Sending flowers as a gift is an age-old practice that never gets old. Countless flowers exist for different occasions, and people have attached meanings to the different flowers. No matter the occasion or incident, there is a flower arrangement for it if you visit any […]
ShoppingSending flowers as a gift is an age-old practice that never gets old. Countless flowers exist for different occasions, and people have attached meanings to the different flowers. No matter the occasion or incident, there is a flower arrangement for it if you visit any florist shops. In this blog, we will share how to send flowers to someone in Brampton.
Here’s how to do it:
- Determine the occasion
Deciding why you are sending them flowers is essential to choosing the right bouquet. The internet has made flower delivery in Brampton very convenient and popular. People send flowers for many reasons or occasions. These include:
- Birthdays
- Congratulations
- Love confession
- ‘Thinking about you.’
- Apologies
- Etc.
Once you determine the reason for sending the flowers, the florists can recommend the best flower arrangements for your purpose. Or you can search online for flowers that symbolize your intentions.
- Think about the recipient’s space
It is hard to know what people do with gifts. But if you’re familiar with this person, you may want to think of where they will keep the flowers when they receive them. Sending a large bouquet to someone who lacks room for it could result in waste. If you’re unfamiliar with the person’s area, then going for a moderately sized assortment would be the best option.
- Choose a good florist
Brampton has more than a few florists and flower delivery businesses. A great way to find a good florist is through customer reviews online. This would give you an idea of their professionalism and reliability. You may not be able to see the flower arrangements before it gets to the recipients. Very few florists allow such. That is why choosing a reputable one with a high customer rating is important.
- Send the flowers
Many florists do all their work online these days. So if you choose one of these, sending the flowers will be quick and convenient. You would simply pick a flower arrangement on their website, fill out their forms, make payment, and they would do the rest. If you have trouble choosing a bouquet, you can talk to them via live chat or phone call. Visiting them is also an option. You would see the actual flowers and talk to someone face to face. Whether you are filling out the online form or seeing them in person, giving the full and correct information they request is crucial. For instance, you would have to explain the occasion or incident. Also, ensure you provide the accurate address. So it is worth reconfirming the recipient’s actual location any way you can to prevent mistakes.
Conclusion
With these steps, you can have flowers delivered to anyone in Brampton and Canada. It is essential that you give the florists all the details they request, including the purpose of sending the flowers. It helps them give you the best recommendations. Reconfirm your recipient’s address also to avoid mistakes. If you order early enough, some florists can have your flowers sent on the same day.

5 Common Mistakes People Make When They are Facing Criminal Charges
Mistakes are the part of life. People make mistakes when facing criminal charges, but we are here for that. We will tell you what NOT to do so you can avail your legal rights and build a strong case for yourself. Criminal law firm Mississauga has seen many people […]
Law & LegalMistakes are the part of life. People make mistakes when facing criminal charges, but we are here for that. We will tell you what NOT to do so you can avail your legal rights and build a strong case for yourself. Criminal law firm Mississauga has seen many people making the following mistakes.
We will tell you how to avoid those so that you will no longer be charged with the crime you did not do. The criminal justice system is not perfect; nothing is; we must stand up for ourselves.
- Resistance to arrest
It is a pretty typical response to resist and try to get yourself free when you are being arrested, you should avoid that. It will make things worse for you and your case if you fight. It could lead to even more charges on your name when trying to free yourself from the previous one.
Be respectful, polite, and calm while dealing with the police. But it does not mean that you tell them everything they ask. Refuse to answer anything, whether it’s a piece of evidence or questions. Your answers can be used against you to strengthen the charges you are facing.
2. Fail to contact a criminal defence lawyer
Whenever you get arrested, ask for a lawyer. If you are not connected to the justice system and don’t know how it works, you should immediately contact your lawyer or tell the police to get you a lawyer before anything else. The lawyer will better be able to protect your legal rights and guide you through the criminal justice system.
Your lawyer can help drop the charges by presenting the evidence supporting you. Although, if you are guilty, your lawyer can negotiate with the prosecution to minimise the penalties or charges. All of this can happen if you become successful in getting yourself a lawyer. The lawyer will be able to represent you in court in case your case leads to a trial. He will know your complete detail from the start and be better able to defend you than yourself.
3. Providing evidence to the police
The police will ask if you have any other evidence. We do not want you to hand this evidence straight to the police. It can be anything, and we advise you not to provide the police with ANYTHING! For example, if the police ask for your body fluid samples or fingerprints, you should ask for a court order before doing that.
Do not hand over your mobile phone passwords, laptop or other personal things that could be used against you.
4. Dressing in a court
This is the time you spend some money on your outfit. You should look presentable and respectful in front of the court. The legal system is working for people, and we should have an excellent physical appearance to be obedient. Just dress tidy and respectful, like wearing a coat and a tie.
5. Hiding information from your attorney
You should never hide information from your attorney, as he will not be able to help you if he doesn’t know the whole story. Enlighten him will all the evidence, suspects, facts or any other detail related to your case.
Conclusion
This article covered what mistakes you should avoid when facing criminal charges. You should never hand away any evidence, your fingerprint, to the police. Most importantly, you should get an attorney as soon as possible to protect your legal rights better. The lawyer will help you get over the Criminal charges or reduce your sentence.

by Randal Spain
When Do You Need A Personal Injury Lawyer? Take This Quiz To Find Out
With so many categories of lawyers out there, victims of accidents can be confused about who to seek legal help from. Well, you may have heard of a personal injury lawyer, but you may not know when to contact one. And in today’s guide, you […]
Law & LegalWith so many categories of lawyers out there, victims of accidents can be confused about who to seek legal help from.
Well, you may have heard of a personal injury lawyer, but you may not know when to contact one. And in today’s guide, you should find the answer to just that.
What Does A Personal Injury Lawyer Do?
Before anything else, it’s important to understand the role of a personal injury lawyer.
As the name may have already suggested, a personal injury lawyer helps you file monetary claims if you’ve been injured due to an accident, typically caused by a vehicle. This claim is actually filed against the negligence of an individual or business which has caused the accident in the first place.
You can take the help of a personal injury lawyer to seek finances for physical, emotional or psychological damages. And these finances can be in the form of medical or legal bills or income lost due to the injuries inflicted by the accident.
Do You Need A Personal Injury Lawyer?
You may think getting in touch with the best Brampton injury lawyers every time you’re injured in an accident is the wisest thing to do. However, that’s not always true; you should seek the services of personal injury lawyers under specific circumstances.
So, take the following quiz and see if you can answer all the questions (or most of them) in the affirmative.
1. Will You Miss Commitments Due To The Injury?
If the injury causes you to miss commitments with regard to work, education or your overall life in general, then it may be a good idea to contact a personal injury lawyer. This can include:
- Staying absent from work or classes due to treatment
- Emotional or mental trauma that prevents you from functioning
- Losing the quality of life you had before the injury
- Medical condition from the injury that stops you from enjoying life
2. Are You Left Permanently Disabled?
Permanent disability, whether physical or mental, is one of the top reasons why accident victims seek legal help from personal injury lawyers. Most such disabilities result in high medical and lifestyle costs, which can be covered by the organization or individual involved in the accident.
And if the accident isn’t caused due to your fault, you’re eligible for a direct concession on medical expenses.
3. Are Your Medical Bills Left Uncovered By Insurance?
Even if you haven’t been permanently disabled in the accident, your medical bills may still be high due to operative or postoperative expenses.
If the accident wasn’t your fault, then there’s no reason why you should bear these costs, especially if the insurance can’t cover them. In such a situation, you can consider hiring a personal injury lawyer.
Final Words
Life is unpredictable, so the minimum you can do is try and stay prepared for unfortunate incidents like accidents and their aftermath.
Hence, we’d suggest doing some research to find out the best personal injury lawyer near you. Make sure to go through their testimonials and the type of injuries they help clients fight for. And don’t hesitate to reach out for a consultation!

Should You Throw Away Your Torn Or Damaged Carpets?
Normally, we prefer to throw away damaged and torn carpets. But instead of throwing them away, you can recycle and reuse old carpets for several purposes. For instance, you can cut the carpet into small rags and use them for cleaning. By doing so, you […]
Home ImprovementNormally, we prefer to throw away damaged and torn carpets.
But instead of throwing them away, you can recycle and reuse old carpets for several purposes. For instance, you can cut the carpet into small rags and use them for cleaning. By doing so, you can save money and the environment in the long run.
Besides this, you can contact a carpet patching company in Toronto to restore your old carpets. On that note, we have put together this guide to help you find ways of reusing old carpets that are damaged and worn out.
So, let’s proceed to the next section!
Four Ways Of Reusing Old And Damaged Carpets
1. Use Old Carpet As Rags
Oftentimes you require a cleaning rag to wipe the windows and floors of your house. Instead of spending money on a new rag, how about using old carpets to make rags for cleaning?
In this way, you can save money since you are reusing the old carpet to make rags. Moreover, the rags will last for a long time since the materials used for making carpets are usually durable.
2. Contact The Local Recycling Organisation
Most carpets are made from materials that can easily be recycled; therefore, throwing them can be a waste since they can be used for something more productive. The local recycling organizations are well equipped to take care of this problem since they can recycle the carpet and use it for different purposes.
For instance, they can make small door mats out of carpets or use them for other sustainable projects.
3. Making Floor Mats For Your Car
If you own a car, you might feel tempted to buy a new floor mat whenever the old one is worn out. However, you can simply reuse the old carpet lying in the house and make floor mats for the car. Not only is it economical, but it will also make your car look as good as new!
Similarly, you can use them to make rectangular door mats for your house entrance and rooms. So, the next time you see an old carpet, simply get a hold of a pair of scissors and make yourself some door mats and floor mats.
4. Making Bed Covers For Pets
Lastly, you can use the old carpets to make bed covers for your dog or cat. Carpets are usually made out of wool. Hence, they can come in handy for keeping your pet warm during the winter.
Conclusion
With that, we have reached the end of this guide. You can contact a carpet repair company if you plan to repair your old carpets and reuse them. Besides this, you can watch different DIY videos on how to reuse old carpets for various sustainable projects.
That said, it’s a wrap!

5 Skincare Essentials You Need To Look Young!
We all want to look our best at all times. However, as we age, it becomes increasingly challenging to maintain a youthful appearance. So, if you are looking to buy beauty products that can turn back the clock and provide youthful-looking skin, you are in […]
Beauty & FashionWe all want to look our best at all times. However, as we age, it becomes increasingly challenging to maintain a youthful appearance.
So, if you are looking to buy beauty products that can turn back the clock and provide youthful-looking skin, you are in the right place! Here are five skincare essentials that will help you fight the signs of aging to achieve radiant and glowing skin.
5 Skincare Essentials You Need To Look Young!
1. Sunscreen
Wearing sunscreen regularly is one of the most important things that can benefit your skin. Not only does it protect your skin from potential damage caused by harmful ultraviolet radiation, it also helps to keep fine lines and wrinkles at bay.
2. Moisturizer
Moisturizing is vital for keeping your skin hydrated and preventing dryness. In addition to nourishing your skin, some moisturizers can protect it from pollution and UV radiation.
Choose a moisturizer suitable for your skin type and use it twice daily, in the morning and at night, after cleansing your face and neck. Try using a hyaluronic acid serum beneath your moisturizer for better results.
3. Exfoliator
Exfoliating is beneficial for dry and aging skin, as it helps remove dead cells. Both store-bought and natural exfoliating ingredients like oatmeal and orange peels can be used for this process. Exfoliating your skin consistently may give you softer, brighter, and firmer skin, reducing the appearance of wrinkles.
And you should know that the best effects will come from exfoliating your skin once every week.
4. Eye Cream
The skin around our eyes is delicate and prone to showing signs of aging before the rest of our face. Luckily, several eye creams can help reduce wrinkles, dark circles, and puffiness. And applying one every night can even hydrate the skin and soothe inflammation.
On that note, here are some tips for choosing an effective anti-aging eye cream. Firstly, look for anti-aging compounds like hyaluronic acid or retinol. Secondly, choose a cream that is suitable for your skin type. And finally, don’t forget to apply sunscreen daily to protect your skin from further damage.
5. Face Masks Or Packs
Most of us are familiar with the word “face pack.” Many of us have probably used it at some time, whether at a spa or home, but what is a face pack exactly? Well, a face pack is generally applied to the face to enhance the complexion.
You will find many face masks and facial kits that contain natural ingredients such as honey, rose water, almond oil, etc., which can be used once a week to tighten and rejuvenate the skin. They may be just what you need to get clean, younger-looking skin.
Final Thoughts
Skin care is essential at any age, but it’s especially crucial to start caring for your skin if you want to look young and fresh for years to come.
Additionally, ensure you drink a lot of water every day as well. Hydration is crucial to keep pimples away. And don’t delay buying your skincare essentials any longer to get back your glow!

5 Tips for Choosing the Right Garage Door for Your Business
Owning a garage in a commercial space without a proper door means you will be practically inviting trouble for yourself. It will give crooks and thieves free rein to deprive you of your precious belongings. And it is needless to say that such an incident […]
Home ImprovementOwning a garage in a commercial space without a proper door means you will be practically inviting trouble for yourself.
It will give crooks and thieves free rein to deprive you of your precious belongings. And it is needless to say that such an incident will negatively affect your business.
That said, many business owners may not be as savvy about garage doors as they are about running a business. For such people, here are some useful tips that can help when selecting a garage door.
5 Tips For Choosing The Right Garage Door
1. Ensure The Correct Size
To choose the best possible door, you should measure the dimensions of your garage entrance before speaking to a commercial garage door supplier (USA).
It is safe to assume that you will store multiple vehicles in your garage, along with the necessary equipment to repair and maintain them. Because of this, your garage will need to be spacious. And a spacious garage will obviously have a much larger entrance, due to which it will need a bigger door.
2. Choose A Durable Material
You would obviously want to make your garage as durable as possible. This will protect it from getting tampered by crooks and weather elements like thunderstorms and snowfall.
Keeping that in mind, the best materials for garage doors are steel and aluminum, as they will last for a long time with little maintenance. However, if you have a tighter budget, you can go with wood or composite garage doors as well.
3. Select A Convenient Style
Garage doors come in different styles, such as sliding, sectional, canopy, swing-out, and roll-up. Each one has its own set of perks, so you can pick whichever you prefer. Although, it is better to go with the sliding and sectional doors since they are slightly more convenient to operate than the others.
4. Make Sure The Garage Doors Are Safe
When choosing a garage door, you should ensure that it has one or more additional features to enhance the overall safety and security of your garage. These features can include anti-tamper mechanisms, remote-controlled operation, security code access, alarms, and so on.
5. Stay Within Your Budget
Finally, the most important tip that you should keep in mind is your budget. It wouldn’t make sense if you spent a large chunk of the business capital just on getting a garage door. You shouldn’t have a small budget either, as you won’t be able to buy a good door with it.
Conclusion
Ideally, for a commercial garage, it’s better to focus more on the practical aspects of the garage door instead of its aesthetics. But if you have the necessary funds, and want your business establishment to look more appealing, then the choice is up to you.
Anyhow, if you have a garage that needs a door, contact your nearest garage door supplier immediately!

How Often Should You Change Your Car’s Oil?
Everyone loves cars. They’re sleek, fast, and powerful but require regular maintenance if you want them to keep running smoothly. Whether you drive an old clunker or one that’s been around for decades, it’s essential to know how often you should change your oil and […]
AutoEveryone loves cars. They’re sleek, fast, and powerful but require regular maintenance if you want them to keep running smoothly.
Whether you drive an old clunker or one that’s been around for decades, it’s essential to know how often you should change your oil and other fluids so your vehicle stays on the road for years longer than it would.
Here’s how often you should change your car’s oil:
Why Do Oil Changes Matter?
Oil is the lifeblood of your engine. It lubricates and cools the engine, keeping dirt and metal shavings out. If you don’t change your oil regularly, it can break down and cause severe damage to your engine.
So why do I need to change my car’s oil every 3,000 miles? Because if you don’t do so, then dirt builds up in there (since you’re not driving much), which causes friction between moving parts—and that’s bad news for any vehicle owners.
You can also use industrial equipment service to learn more about this.
How Often Should You Change Your Oil?
The frequency of oil changes depends on several factors, including the type of vehicle and how it is driven. For example, an engine with a high compression ratio needs more frequent oil changes than a lower one.
Also, consider whether your car’s engine uses synthetic or regular motor oils.
Synthetic motor oils are better for engines that run at higher temperatures; they have additional additives that help reduce friction in hot engines by breaking down tar deposits during operation (which can cause overheating).
What Kind of Oil Should I Use?
If you’re wondering what oil to use in your car, there are a few things to consider.
First and foremost, you should ensure that the oil is compatible with your vehicle type.
For example, suppose your car has an engine with turbochargers or diesel engines (which can be found on many modern vehicles).
In that case, it’s likely better to use synthetic oils instead of conventional ones.
When Should I Change My Oil Filter?
The oil filter is a critical part of your engine. It’s where contaminants get trapped and prevent proper lubrication, damaging the entire engine.
If you don’t regularly change your oil filter, you’re putting yourself at risk for expensive repairs or even total failure of your vehicle.
While it might seem like a hassle to change an oil filter every time you replace the engine oil (and sometimes even more often), this task is relatively simple and only takes about five minutes per vehicle.
Verdict!
The best way to determine how often you should change your car’s oil is to take it to a professional. They can tell you precisely what oil is best for your vehicle and how often to change it. And remember, if you need an oil change, don’t forget the other essential services listed above!

Everything You Need to Know About Roofing Warranties?
When it comes to roofing warranties, there seems to be a lot of confusion among homeowners. What is covered under a roofing warranty? How long does the warranty last? What happens if there is a problem with my roof after the warranty expires? Below, we […]
Home ImprovementWhen it comes to roofing warranties, there seems to be a lot of confusion among homeowners. What is covered under a roofing warranty? How long does the warranty last?
What happens if there is a problem with my roof after the warranty expires? Below, we will demystify roofing warranties and answer all your questions. Let’s get started!
What is a Roofing Warranty, and What Does it Cover?
A roofing warranty is a document that outlines the terms of coverage for your roof. It typically includes information about what is covered, how long the coverage lasts, and who to contact if something goes wrong. Most importantly, a roofing warranty should give you peace of mind that your investment is protected.
How Long Do Roofing Warranties Typically Last For?
The length of a roofing warranty depends on the type of materials used and the manufacturer. Some warranties only cover material defects, while others also cover workmanship defects. Typically, material warranties last 20-50 years, while workmanship warranties only last 1-5 years.
What Happens if Something Goes Wrong with My Roof after the Warranty Expires?
If something goes wrong with your roof after the warranty expires, you will likely have to pay for repairs or replacement out of pocket. That’s why choosing a reputable contractor and high-quality materials are essential – to increase the chance that your roof will stand the test of time.
Are There Any Other Types of Warranties I Should Be Aware of When Getting a New Roof Installed?
In addition to material and workmanship warranties, some contractors also offer labor warranties. Labor warranties typically cover 1-5 years and protect against poor workmanship or faulty installation.
How Can I Make Sure I’m Getting the Most Out of My Roofing Warranty?
There are a few things you can do to make sure you’re getting the most out of your roofing warranty:
- Choose a reputable contractor with a proven track record
- Select high-quality materials from a trusted manufacturer
- Make sure all paperwork is completed and filed correctly
- Keep detailed records of any repairs or maintenance performed on your roof during the warranty period
By following these tips, you can be confident that your roof is covered in case anything goes wrong.
Verdict!
A roofing warranty is an important document that every homeowner should be familiar with. In this blog post, we’ve answered some common questions about roofing warranties so you can be an informed consumer. We hope this information has been helpful and gives you peace of mind knowing that your investment is protected.
If you are unaware of the benefits of attic insulation or looking for roofing experts to get help then reach out to us. We’d love to help.
FAQ’s
Q1: What is a roofing warranty?
A: A roofing warranty is a document that outlines the terms of coverage for your roof. It typically includes information about what is covered, how long the coverage lasts, and who to contact if something goes wrong.
Q2: How long do roofing warranties typically last for?
A: The length of a roofing warranty depends on the type of materials used and the manufacturer. Some warranties only cover material defects, while others also cover workmanship defects. Typically, material warranties last 20-50 years, while workmanship warranties only last 1-5 years.
Q3: Are there any other warranties I should be aware of when installing a new roof?
A: In addition to material and workmanship warranties, some contractors also offer labor warranties. Labor warranties typically cover 1-5 years and protect against poor workmanship or faulty installation.

6 Types of Garage Door Materials that You Need To Know
Choosing a garage door is one of the most important decisions you will make for your home. Not only does it add value to your property, but it also plays an important role in determining how people perceive your home. So if you want to […]
Home ImprovementChoosing a garage door is one of the most important decisions you will make for your home.
Not only does it add value to your property, but it also plays an important role in determining how people perceive your home.
So if you want to make sure that your new door is going to stand out from others, then it’s worth taking some time to consider what materials are best suited for different uses.
Traditional Steel
Steel garage doors are the most common and widely used material. They’re durable, but they can rust if not properly cared for. Steel is usually painted with a baked on enamel finish that will protect it from the elements.
Steel garage doors come in many different colors and finishes to match your home’s decor or personality. You can find steel door options in reds, oranges, blues, greens—you name it!
Wood
Wood is a common material for garage doors. The most popular style is the traditional wooden panel or double-hung, which consists of two panels that swing up and down.
You can also choose from other options like tongue-and-groove boards, slat systems and even vinyl replacement panels that feature a textured finish for an eye-catching look.
Modern Aluminum
Aluminum is a lightweight, rust-resistant material. It’s also one of the best options for garages with high humidity or frequent exposure to the elements, like those in your home.
Aluminum can be painted or powder coated in any color you choose—including white, black and stainless steel finishes. This makes it an affordable option compared to other materials such as fiberglass or steel which require more expensive paint jobs to match their appearance aesthetically speaking!
Fiberglass
Fiberglass is a composite material made of glass fibers and resin. It’s strong and durable, resistant to fire, rot, and insects. It comes in white, black, brown and tan.
Glass
Glass garage doors are a great choice for homes that have high-end interiors. They’re also more expensive than other materials, but they have many advantages over vinyl and wood.
Glass garage doors let you see your car from inside the house, which is particularly convenient if you have an open concept living space or large dining room. The glass also makes it easy to clean because dirt doesn’t get trapped behind the door as easily (you can just wipe down with a damp cloth).
Wood Alternatives
Wood alternatives are made from recycled plastic and can be used with steel or aluminium tracks. They may be painted to match your home, but they don’t come in a wide variety of colours.
The biggest benefit of wood alternatives is that they’re easy to install: You simply need to screw the opener onto one end of each sectional door and then attach it to your garage flooring.
Wood alternative doors work well for people who want an attractive appearance without paying top dollar for high-quality materials like fibreglass or steel—but if you prefer more luxury than bargain basement pricing, these options aren’t for you!
Verdict!
Learning about garage doors such as garage door types, materials or learning how to level the garage doors using different materials. But don’t worry, we have curated a list of 6 garage door material types that you must know as it will help you choose the right material in future.

Types of Patents that You Need to Know!
Patents are an essential part of intellectual property law. They give you a legal right to prevent others from using your invention without permission. In today’s digital era, patents don’t just protect new inventions—they also help protect existing products. For example, Apple has been using […]
Law & LegalPatents are an essential part of intellectual property law. They give you a legal right to prevent others from using your invention without permission.
In today’s digital era, patents don’t just protect new inventions—they also help protect existing products.
For example, Apple has been using its patented design for the iPhone since 2007 and therefore has strong patent protection against knockoffs (like clones).
Utility Patents
A utility patent protects the way something works. This is the most common type of patent, and it’s also called a process patent because it covers an invention that makes use of existing processes or machines to perform some function in a new way.
The United States Patent and Trademark Office (USPTO) considers utility patents more important than design patents because they can be used to stop competitors from selling similar products at lower prices. If you want your product or service to be successful, you need to protect yourself against copycats by getting as many utility patents on it as possible—and fast!
Design Patents
Design patents protect ornamental designs of useful objects. The design must be new and non-obvious, which means that it can’t have been used before in any other way. It also must be applied to a useful object, so if you make a pair of socks with your new twist on the traditional heel, they will not be eligible for design patent protection unless they qualify as an ornamental design.
The scope of this type of patent protection is rather broad: it protects any “new” or “useful” ideas, whether they’re novel or old hat—and if you’ve got something new and useful on deck right now (or even just something old), then it might be worth checking out what protections are available through this route before moving forward with your next big project!
Plant Patents
Plant patents are used to protect plants that are asexually reproduced, and they have a 20-year term.
This means that if you want to own a plant patent on your favorite variety of tomato, you can register it with the U.S. Patent and Trademark Office (USPTO) at any time during its lifetime—even after someone else has already registered their own version of the same type of tomato!
Having Patents is Crucial to Protecting Your Intellectual Property
Having patents is crucial to protecting your intellectual property. Intellectual property is a form of intangible asset that you can register with the government, which gives you the right to prevent others from using or selling the ideas for which you hold patents.
For example, if someone came up with an idea for a new type of computer chip and patented it, they would be able to prevent anyone else from making or selling this part.
Patents also protect other forms of intellectual property such as copyrights and trademarks (which we’ll talk about later).
Verdict!
It’s important to know that patents are not just for inventors or creators. They can protect your ideas, too! While the tech world is flooded with patents for new products, those in other industries might not have access to any at all – even though their inventions could be just as valuable. If you’re struggling to come up with a good idea and want some help getting it patented, call our patent agency today!

5 Mistakes You’re Making With Hcg Drops
Nowadays, most people prefer to lose weight and maintain their health. HCG drops are a supplement that can help you lose weight quickly. However, HCG drops are not entirely safe, and it does come with some health problems. As such, we have compiled this guide […]
Business FitnessNowadays, most people prefer to lose weight and maintain their health. HCG drops are a supplement that can help you lose weight quickly.
However, HCG drops are not entirely safe, and it does come with some health problems. As such, we have compiled this guide to help you understand the five mistakes people make while purchasing online HCG Drops atlanta. So, without further ado, let’s proceed to the next section!
Five Mistakes People Make With HCG Drops

1. Not Preparing A Meal Plan
One of the most common mistakes people make when they start taking HCG drops is that they don’t plan their meals properly. HCG drops have certain dietary restrictions which prevent people from consuming particular food items.
As such, if you don’t plan the meal routines ahead of time, you might face a lot of problems finding the right food to suit the supplement. Plus, your meals can turn up boring if you don’t plan ahead of time. Proper research can help you find ways of making the meal tasty as well as nutritious.
2. Excessive Exercise
Most people often have a misconception that excessive exercising and working out can help the HCG drops to cut down more weight. However, this isn’t true because HCG drops take a long time to show results.
Instead, we recommend following a moderate exercise routine that suits your daily schedule. Avoid excessive work out since that can add a lot of stress to your muscles and cause injuries.
3. Not Drinking Sufficient Water
Another common mistake people make is that they assume water can hinder the weight loss process. As such, people avoid drinking plenty of water since they want to lose weight faster by taking HCG drops.
But the truth is that water plays an important role in losing weight since it helps to flush out harmful toxins and waste from the body. Moreover, if you don’t drink plenty of water, your body will retain excess water, and this can increase body weight. Thus, you should always drink plenty of water to avoid unnecessary health complications.
4. Stopping HCG Drops Too Soon
If you want the HCG drops to show real results, you must always follow the plan given by the dietitian or the expert. Stopping the dose too early can harm your body since it wouldn’t be able to adapt to the sudden change in hormones, and this can have an adverse effect on your body.
5. Avoiding Certain Foods Entirely
Just because you are on an HCG diet plan, it doesn’t mean you have to avoid a certain food entirely. In addition, most people follow a boring meal routine in which the same food is repeated over and over again.
As such, you will lose out on a lot of nutrition, which your body will require to function optimally. It can also cause an imbalance of certain vitamins in the body which can lead to several health problems. Thus, we recommend following a balanced diet in which certain food items are eaten in moderation.
Conclusion
To conclude, HCG drops can help you lose weight, provided you maintain a strict diet routine set by your dietitian. But this shouldn’t stop you from enjoying your favourite treats once in a while. In fact, you can always look for recipes online to make your daily diet interesting and tasty. And if you have any further doubts, we recommend contacting the local store that supplies HCG drops.

5 Pros And Cons Of Vaping You Must Know
Vaping has become all the rage among the youth of today. They consider it to be a much better and trendier alternative to conventional smoking. For the most part, they are correct since vaping liquids do not contain most of the harmful chemicals present in […]
FoodVaping has become all the rage among the youth of today. They consider it to be a much better and trendier alternative to conventional smoking.
For the most part, they are correct since vaping liquids do not contain most of the harmful chemicals present in a traditional cigarette. Besides, vaping provides more freedom of choice to the user than conventional cigarettes, which is a very appealing aspect.
That said, vaping still has its risks, so it needs to be done in moderation. Keeping that in mind, this guide has discussed the different pros and cons of vaping. If you are curious, then read on!
5 Pros Of Vaping
1. Safer Than Regular Smoking
Conventional cigarettes contain tobacco, which is composed of numerous harmful chemicals. Since vaping involves little to no tobacco, it is safer in comparison.
2. Control Over Nicotine Intake
The vaping liquid used in an e-cigarette comes in different nicotine strengths. This gives you complete control over how much nicotine you are smoking.
3. Highly Accessible
You can purchase e-cigarettes, liquids and other vaping tools from an online vape store (Canada) as well as offline channels. What’s more, they are quite affordable, which makes them easily accessible to everyone.
4. Comes In A Variety Of Flavours
Vaping liquids are available in a wide range of flavours, such as raspberry and strawberry. So, you can pick whichever option suits your palate the most.
5. Helps To Quit Smoking
If you are a long-time smoker who is worried about the destructive effects of this habit, you can take up vaping as an alternative. It feels the same as smoking, minus many of the drawbacks, so you can use it to let go of your habit over time.
5 Cons Of Vaping
1. Health Risks
While it doesn’t contain tobacco, it does contain nicotine, which has a lot of harmful effects, especially when taken in large amounts. Besides, it has been observed to cause other health problems, such as depression or even stroke, among others.
2. Leads To Addiction
The nicotine present in vaping liquids is addictive in nature. So, if you do not keep your vaping activities in control, you will get addicted to it.
3. Has A Learning Curve
If you want to get the best experience out of vaping, you will need to start with a simple beginner’s product. As such, it has a learning curve which many users may find frustrating.
4. A Growing Problem Among Teenagers
A large number of teenagers today have easy access to vaping. They are particularly vulnerable to the destructive effects of nicotine and might develop an addiction to it eventually, which is a cause for concern.
5. The Vaping Liquids And E-Cigarettes Can Catch Fire
The e-cigarette used for vaping can easily catch fire and, in extreme cases, may explode in your hand. Similarly, the vaping liquid is highly flammable, making it risky to use.
Conclusion
Nobody has ever said that vaping as a habit is absolutely safe. But if you keep it under check, then vaping is indeed a much better alternative to smoking.
So, if you are interested in getting into it, visit a vape store today!
More Stories


How to Boost Sales Using Experiential Marketing
Experiential marketing continues to grow in popularity because of its effectiveness. Businesses use it to interact directly with customers, establishing and maintaining strong customer relationships. Therefore, you are on the right track if you are considering an experiential marketing campaign. However, the only way to […]
TechnologyExperiential marketing continues to grow in popularity because of its effectiveness. Businesses use it to interact directly with customers, establishing and maintaining strong customer relationships. Therefore, you are on the right track if you are considering an experiential marketing campaign. However, the only way to use it to generate favorable results is if you do it right. Below, we explain the basic steps to using experiential marketing to boost sales.

Step 1. Develop in-depth knowledge of your consumers
Knowing your customers’ sex, age, and demography is good enough. But launching a successful marketing campaign requires more. Understanding their interests and buying habits helps you plan an effective campaign that resonates with them. An experiential marketing professional can help design strategies to determine these elements.
Step 2. Determine the campaign goal
Every marketing campaign must have a goal. Once you define the actual goal, it helps you establish the Key Performance Indicators (KPIs) and measure the campaign’s success. Examples of good KPIs for experiential marketing are the number of people who participated, the number of people who willingly continued the engagement, the number of sales conversions, etc.
Step 3. Prepare a clear message for the campaign
A successful marketing campaign consists of clear communication of your brand’s voice. Ensure the message is clear for the customer without any contradictions. Let the message align with what they already think about the brand. The participants should have fun during the activities, and also they should understand the relevance of the message to your brand.
Step 4. Determine the platform
Decide if you want an offline or online event once you have set the goal and the message. Offline events let customers get involved in the service or product. Many companies offer free giveaways to encourage walk-in customers during the event. However, you’ll need more resources in terms of staff and promotional items. Online, on the other hand, may require fewer resources. In today’s internet and social media era, customers are always online and use it to search for products. For this reason, marketers are now finding ways to get customers to test their products rather than just seeing their ads.
Step 5. Design your engagement plan
Experiential marketing is about getting customers involved in your product or service. It is about engaging their senses, either sight, taste, smell, sound, or touch. The engagement should propel a behavior change through new experiences, creating a lasting impression. The engagement should call up pleasant emotions. That is why this form of marketing is so powerful and effective. The event must not only create brand awareness, but make the customers feel good while interacting with your brand, product, or service.
Step 6. Launch the campaign
Once you have made all the preparations, it is time to launch the campaign. Ensure you record the event to review it later. If it is an offline event, you can take photos and videos. If online, use trackers to document the engagement.
Experiential marketing lets people experience your product or service and get involved in your brand. Through this, you earn an important place in the customer’s mind, consequently boosting your revenue.

Your Ultimate Starter Guide to Virtual Counseling Services
Counseling services have become convenient with the availability of online counseling services. The restrictions imposed due to the pandemic accelerated the use of virtual counseling. If you are new to virtual counseling, here is a simple step-by-step process to get you started. 1. Understand if […]
BusinessCounseling services have become convenient with the availability of online counseling services. The restrictions imposed due to the pandemic accelerated the use of virtual counseling. If you are new to virtual counseling, here is a simple step-by-step process to get you started.
1. Understand if online counseling works for you
Online counseling has many advantages when compared to traditional sit-ins with a counselor. It saves time and finances you would have used to get to a counselor’s office. Furthermore, you can have the counseling session anywhere, at home or in your office.
Some people prefer talking with their counselors face to face. Others may not feel connected to their counselor when having an online session. Before you register and start counseling, find out if it is the right choice.
2. Find a virtual counseling service
Many counseling clinics offer online therapies. You can find the different services by searching for their websites online. You can see the pricing and the different types they offer on their website and compare them.
Check the reviews and recommendations for the virtual counseling clinic. Use the contacts to get more information about the counseling clinic. You can also request the qualifications and licensing of the counselors available.
Ask how you can pay for the sessions and if they accept your insurance provider. Request to know how they conduct their online sessions. After looking at different options, choose from the best virtual counseling service.
3. How secure are virtual counseling services?
Virtual counseling must be confidential and secure to protect client privacy. They use encryption on private meetings or video and phone calls. You also have to ensure your devices are secure. Get a safe place to express your feelings.
4. How do I prepare for an online session?
Start by finding a soundproof, secure pace or where you can talk. Find enough time for the counseling session. Prepare your mind for the session and after the session, reflect on the issues discussed.
If it’s your first therapy session, ask questions like;
- How long does the session take?
- How can I cancel a session?
- How many sessions will you need?
- What will you need for the therapy session?
- What other methods can you use in case of a technical problem?
- How does the counselor guarantee confidentiality on his side?
These questions will help to prepare and reduce anxiety during the first session. They also assist patients in understanding how the counseling session happens.
Pros of virtual counseling services
- It has a variety of methods, such as texts, email, videos, and calls
- It is efficient for busy individuals
- It is efficient as you can get your counselor using your devices at home or work
- It does reduce the cost you would have used to get to the counselor’s office
Cons
- It relies on technology and WI-FI
- Most virtual counseling services do not accept insurance payments
Final words
Virtual counseling services are a game changer when it getting professional help. It has allowed more people that would shy away from a face-to-face session to find help online. If you are looking for a way to get help, use the guide to help you start your journey toward recovery or treatment.

Why Should Your Business Hire Retail Professionals
Retail professionals can help you with a lot of things. They can provide you with the best products, services, and discounts. They can also help boost sales and revenue by ensuring everything is adequately stocked and that customers get what they need when visiting your […]
BusinessRetail professionals can help you with a lot of things. They can provide you with the best products, services, and discounts. They can also help boost sales and revenue by ensuring everything is adequately stocked and that customers get what they need when visiting your store. Let’s take a look at the reasons why your business should hire a retail professional:

Retail professionals are dedicated to the success of your business.
One of the best reasons to hire retail professionals is that they are dedicated to the success of your business. They want you to succeed, and they’re not just looking for a paycheck; they want their clients’ businesses to thrive.
The end goal is always important when making decisions like this—you want someone who will put their time and energy into ensuring things go right every time!
Retail professionals can help you organize your business.
They can help you eliminate waste and inefficiency, saving time and money for everyone involved in running a retail business.
You’ll be able to focus on what matters most by having someone else take care of the day-to-day activities while allowing them time to focus on improving customer service and other aspects of their job duties.
Retail professionals have connections with other professionals.
Retail professionals have connections if you’re looking for professionals to help with your business. For example, if a retail professional knows someone who would like your business’ products or services, they may be able to connect with them.
Retail professionals can give you unbiased advice.
When you hire retail professionals, they can give you unbiased advice on all the significant issues in any business—from hiring staff members and managing inventory to setting prices and marketing strategies.
When you hire retail professionals to handle your payroll, they can help you avoid potential problems. They know what it takes to run a successful business and can give you unbiased advice.
Retail professionals can also help your business grow. For example, if you have struggling employees, they can help you develop a plan to help them succeed. This is important because it can save your company money in the long run.
Retail professionals understand customer service.
Retail employees have an intrinsic understanding of the customer experience, which allows them to provide exceptional customer service. They understand what makes customers tick and how they’re likely to respond to different situations. This can help you avoid common problems and improve your business.
Retail professionals ensure the high quality of your business products.
Retail professionals ensure that your products are accurately priced, high quality, and on time. Whether you’re dealing with a large chain or an independent store, your customers deserve to have all three elements in place every time.
Conclusion
Retail professionals are the backbone of any successful retail business. Retail professionals can help your business succeed, and you should seriously consider hiring them. They have the experience to get the job done efficiently and effectively, as well as connections that can help you find other professionals who can provide additional services for your business needs. The best part is that they’re already experienced in these fields, so there’s no need for training or even long-term contracts to boost the sales of your retail business.

The Top 8 Benefits of a Chemical Peel Treatment
Chemical peels are a cosmetic treatment that can use chemical solutions to remove the top layer of skin. This can be done for various reasons, such as to improve the appearance of wrinkles, scars, or other blemishes. While there are many different chemical peels, they […]
Beauty & FashionChemical peels are a cosmetic treatment that can use chemical solutions to remove the top layer of skin. This can be done for various reasons, such as to improve the appearance of wrinkles, scars, or other blemishes. While there are many different chemical peels, they all work by exfoliating the skin. This process can benefit your skin, which is why chemical peels are becoming increasingly popular. This article will explore the top 8 benefits of chemical peel treatment. From improving your skin’s texture to unclogging pores, learn how a chemical peel could benefit you.
The top 8 benefits of a chemical peel
A chemical peel is a popular cosmetic procedure that can improve the skin’s appearance. Chemical peels have a variety of different types, each with its own benefits. Here are eight benefits of a chemical peel:
Improves the Appearance of Fine Lines and Wrinkles
Chemical peels are a great treatment option if you want to improve your skin’s appearance and the way it feels. A chemical peel can help to exfoliate the skin and reduce the appearance of wrinkles.
Treats Acne
Another common reason people get a chemical peel is to treat Acne. A chemical peel can help to unclog pores and reduce the inflammation associated with Acne.
Reduces Sun Damage
If you have sun damage, you may be considering a chemical peel. A chemical peel does the following: it removes damaged skin cells and leaves healthy new cells to take their place.
Improves Skin Tone and Texture
If you have uneven skin tone or texture, a chemical peel may help. A chemical peel can help to exfoliate the skin and improve its overall texture and tone.
Reduces age spots or freckles
If you have age spots or freckles, consider a chemical peel as a treatment option. A chemical peel can help lighten these pigmentation areas and even out your skin tone.
Minimizes pores size
If you have large pores, consider a chemical peel treatment option. A chemical peel can help to unclog pores and reduce their overall size.
Improves the appearance of scars
If you have scars that you would like to improve, a chemical peel can help. The peel will remove the top layer of scarred skin, revealing the healthy skin underneath.
Boosts collagen production
When we age, our bodies produce less collagen which can result in wrinkles and fine lines. A chemical peel can help stimulate collagen production, which can help improve your skin’s appearance.
How often should you get a chemical peel?
Chemical peels are a skin treatment that improves the appearance and texture of your skin. Three levels of strength differ on how much of an impact they can have on your skin. There are three levels of strength, which differ on how much of an impact they can have on your skin.
You can get a light chemical peel every few weeks or a deep chemical peel once a year. The frequency of your treatments will depend on your skin type, concerns, and goals.
Conclusion
A chemical peel can offer many benefits, from improving the appearance of sun-damaged skin to unclogging pores. If you are considering a chemical peel, consult a board-certified dermatologist or plastic surgeon to ensure that you are getting the best possible treatment for your needs. And remember, always use sunscreen after a chemical peel to help protect your newly peeled skin!

7 Customer Service Danger Signs When Shopping for Online Vape Shops in Canada
Shopping for e-cigs online in Canada has its merits and demerits. One of its biggest merits is comparing various products and prices before buying. Plus, you don’t need to go out of the house. So it’s highly convenient. But there are demerits too, and an […]
ShoppingShopping for e-cigs online in Canada has its merits and demerits. One of its biggest merits is comparing various products and prices before buying. Plus, you don’t need to go out of the house. So it’s highly convenient. But there are demerits too, and an important, avoidable one is poor customer service. It is tough to determine this at the early stage if you shop online for vapes in Canada. But there are ways to tell the service quality, and this blog shares 7 signs of poor service quality in an online vape shop.
1. Non-compliance with vape regulations
Vape regulations differ in each province and municipality. However, there is a common law among them, which is the age requirement. A good online vape shop in Canada complies with this by ensuring site visitors confirm they are old enough. A pop-up appears on the screen of such a website requesting age confirmation before viewing the site information. The legal age in most parts of Canada is 19. So the pop-up message asks you to confirm you are 19 or older before the page loads
2. Bad reviews or low rating
Another sign of poor customer service is when you find several bad comments about the vape shop. Or if they are rated one or two stars on a trusted review website, e.g., Trustpilot. If only one out of two or three persons gave a low rating or bad comment, this is not enough to judge. However, if there are several low ratings among many reviewers, you should rethink shopping with them.
3. Inadequate details about vaping products
Shopping for e-cigarettes online provides the convenience of checking different products and comparing them without feeling pressured to buy a certain type. Comparisons require enough information about the products. So each catalog should provide these details.
4. Low customer service standards
An online vape shop should have adequate infrastructure for great customer service. Shoppers want to be able to reach a support team at any time. For this reason, most websites now have live chats. Not only that, but they also offer other means to reach the support team, such as email and phone.
5. Limited variety of vaping products
Online vape shops usually offer a wider variety of e-cigarette products. They can invest more in the products since they aren’t paying for a physical store. Most also use dropshipping and other third-party solutions to raise their inventory. Therefore, it is a red flag if an online vape shop does not offer variety and enough options for choice.
6. Unclear warranty details and return policies
Product warranties are supposed to boost customer satisfaction. Each product’s warranty details and return policies should be clear. This is a sign of quality service. The shop lets the buyer know what they are getting into before they complete the purchase.
7. Over-priced products
Vapes are usually lower priced in online shops than in local ones. Since their overhead costs are lower than local shops, they can sell at reduced prices. These stores are more concerned with selling more products and establishing a solid relationship with customers. An online shop that sells at a higher price than the local store or other online shops is not very customer-friendly.

What Are the Licensing Requirements for a Septic System Installation Company?
If you’re looking to start a septic system installation company, you may wonder what the licensing requirements are. Although septic systems are not as standard in urban areas, they are still used in many parts of the country. There are two main types of septic […]
AutoIf you’re looking to start a septic system installation company, you may wonder what the licensing requirements are. Although septic systems are not as standard in urban areas, they are still used in many parts of the country. There are two main types of septic system installation companies: those that install systems and those that maintain them. Each type of company has different licensing requirements. This article will explore the licensing requirements for both types of companies. We will also provide some tips on how to get started in the septic system installation business.

What does a septic system consist of?
A septic system is a self-contained, off-grid sewage treatment system. Septic systems are most commonly used in rural areas with no central sewer system. Septic systems typically have three components: a drain field, a septic tank, and soil. Wastewater from your home flows into the septic tank, where bacteria treat it. The treated wastewater then flows through the drain field, which is further treated by soil bacteria before finally being discharged into the groundwater.
What are the licensing requirements for a septic system installation company?
There are a few licensing requirements for septic system installation companies. The first is a business license, which can be obtained from the city or county in which the company is located. The second is a septic system installer’s license, which is required in most states. This license can be obtained from the state where the company is located. The third requirement is a surety bond, which guarantees that the company will perform its work by the contract and regulations.
What are the benefits of having a septic system installed?
If you are considering having a septic system installed, consider several benefits. One of the main benefits is that it can save you money on your wastewater bill. This is because the septic system will treat the wastewater before it enters the municipal sewer system. Additionally, having a septic system can help protect the environment by reducing the pollution that goes into our waterways.
What are the drawbacks of having a septic system installed?
There are several drawbacks to having a septic system installed. First, the initial cost of installation can be expensive. Second, septic systems require regular maintenance and cleaning to function correctly. Third, septic systems can fail and cause environmental pollution if not appropriately maintained. Finally, septic systems can attract unwanted pests, such as rodents and insects.
Conclusion
To become a septic system installation company, specific licensing requirements must be met. In most states, a septic system installation company must have a general contractor’s license or a specialized license to perform work on septic systems. In addition, the company must have liability insurance and workers’ compensation insurance to protect its employees and customers. Finally, the company must have a business license to operate legally. Meeting these requirements can be daunting, but becoming a licensed septic system installation company is essential.

How Massage Therapy Is Beneficial For Back Pain?
Back pain is a common issue affecting millions of people worldwide. It can be caused by various things, from poor posture to an injury or even an underlying medical condition. Fortunately, massage therapy has been proven to help ease back pain and improve one’s overall […]
FitnessBack pain is a common issue affecting millions of people worldwide. It can be caused by various things, from poor posture to an injury or even an underlying medical condition.
Fortunately, massage therapy has been proven to help ease back pain and improve one’s overall quality of life.
Let’s take a look at how massage therapy works for those suffering from back pain and the benefits that it offers.
What is Massage Therapy?
Massage therapy is a type of bodywork that involves applying pressure to the muscles and soft tissues to relieve tension and promote relaxation.
Massage therapists use their hands, elbows, forearms, and tools, such as hot stones or heated towels, to provide targeted relief.
Many different types of massage therapies are available, including Swedish massage, deep tissue massage, trigger point therapy, sports massage, aromatherapy massage, lymphatic drainage massage, reflexology, and more. Each type has its own benefits depending on the patient’s needs.
How Does it Work for Back Pain?
When treating back pain with massage therapy, therapists focus on specific areas affected by the pain.
They use kneading or effleurage (light strokes) to target these areas and help reduce tension in the surrounding muscles.
Loosening tight muscles and increasing blood flow to the area can alleviate discomfort while improving the range of motion and flexibility.
In addition to this targeted approach to back pain relief, massage therapy can also help reduce stress levels which can often contribute to or exacerbate existing back problems.
Is Massage Therapy Really Beneficial for Back Pain?
The short answer is yes! Studies have shown that regular massage treatments can be highly effective for those suffering from chronic back pain due to conditions like sciatica or herniated discs.
Additionally, massages can help improve sleep quality which is important for your body’s natural healing process.
Benefits Of Massage Therapy For Back Pain Sufferers
Here are some of the benefits of massage therapy for back pain sufferers:
- Improves range of motion & flexibility
- Reduces muscle tension & stiffness
- Alleviates pain & discomfort
- Stimulates blood flow & circulation
- Improves physical performance
- Enhances relaxation & reduces stress levels
Verdict!
There are numerous benefits associated with using massage therapy as a form of treatment for those suffering from chronic back pain.
By targeting specific areas with various techniques, such as kneading or effleurage, therapists can reduce muscle tension, stiffness, and inflammation, improve the range of motion, stimulate circulation, and enhance relaxation.
Ultimately leading to greater overall health satisfaction, happiness, and well-being!
So, speak with an experienced physiotherapist about it – we’re sure you won’t regret it!
If you are suffering from chronic back pain and looking for an experienced clinic, then brampton physiotherapy clinic is the place where you should consult as they treat such kinds of chronic back pains effectively.

Should You Choose a Vape Without Nicotine?
Vaping has become increasingly popular as an alternative to smoking cigarettes. Many people now opt to use a vaporizer instead of smoking, but did you know that you can also vape without nicotine? In this article, we’ll explore the pros and cons of vaping without […]
AutoVaping has become increasingly popular as an alternative to smoking cigarettes. Many people now opt to use a vaporizer instead of smoking, but did you know that you can also vape without nicotine?
In this article, we’ll explore the pros and cons of vaping without nicotine so you can make an informed decision.

Why is Vaping Harmful?
It’s no secret that cigarettes contain thousands of toxic chemicals that can greatly damage your health. This is why so many people are now turning to vaporizers as an alternative form of smoking.
However, one factor that many don’t consider is whether they should choose a vape without nicotine. It’s important to consider all your options before deciding what kind of vaping you want to do.
Pros and Cons of Vaping Without Nicotine
When choosing a vape without nicotine, there are pros and cons that you should consider before making your decision. Let’s take a look at some of them.
Pros
- No Nicotine: One of the main benefits of using a vape without nicotine is that it has no nicotine-related health risks. This means you get all the flavor and aroma from the e-liquid without any harmful side effects of nicotine consumption.
- Less Addiction Risk: Vaping without nicotine also carries less addiction risk than traditional vaping because no chemical in the e-liquid could lead to addiction or dependence on regular use.
- Flavor Variety: Without nicotine, vapers have more room to experiment with different flavors and aromas since they don’t need to worry about how the addition or subtraction of nicotine will affect their experience.
Cons
- Less Throat Hit: One downside to vaping without nicotine is that it may not provide as much throat hit (or sensation on the back of the throat) since there is no chemical present in the e-liquid to cause it.
- Lower Densities Possible: As mentioned above, when vaping without nicotine, there may be lower densities possible which could lead some vapers to want more intensity from their vapor production than what would normally come from using flavored e-liquids with lower PG ratio values or higher VG ratios.
Verdict!
Whether or not you choose to vape with or without nicotine is ultimately up to you, but considering all the pros and cons beforehand can help ensure that you make an informed decision when deciding for yourself or for another person who may be curious about vaping but unsure if they should take part in it due to potential health concerns related to nicotine consumption.
Ultimately, every individual should weigh their pros and cons before making any decisions related to vaping – but being aware of all available options available in the online vape shop market certainly never hurts!

What is Independent Legal Advice & When is it Used?
The concept of independent legal advice has become increasingly important in recent years, particularly with the changing legal landscape. But what exactly is independent legal advice, and when is it used? In this blog, we will explore the answers to these questions to help you […]
Law & LegalThe concept of independent legal advice has become increasingly important in recent years, particularly with the changing legal landscape. But what exactly is independent legal advice, and when is it used?
In this blog, we will explore the answers to these questions to help you understand when to seek independent legal advice.
What is Independent Legal Advice?
Independent legal advice means that a lawyer not associated with either party involved in a dispute or agreement provides their opinion on a given matter.
This can give each of the parties involved more confidence that the advice they receive is unbiased and based on an objective assessment of the situation.
Independent legal advisors are often brought in to mediate disputes between two parties and provide impartial insights into what would be best for everyone involved.
Furthermore, they could provide recommendations on how both parties could agree on a mutual resolution without resorting to lengthy and costly court proceedings.
When Is Independent Legal Advice Used?
One common instance where independent legal advice may be used is when two parties enter a contract together.
An independent lawyer can help ensure that all clauses in the contract are fair and reasonable for everyone involved, ensuring there are no potential issues down the line due to misunderstandings or loopholes in the language used within the document itself.
This can prevent costly litigation later on, which could ultimately lead to major financial losses for all those involved if not addressed properly beforehand.
Another time when independent legal counsel may be useful is during settlement negotiations between two or more parties.
Disputes over contracts, property ownership, or other forms of compensation can get complicated quickly and require an expert’s opinion for each side’s best interests to be taken care of truly.
Respective lawyers from both sides can provide insight on whether proposed solutions within a settlement offer are fair and aligned with existing laws or regulations concerning the case at hand, giving each individual greater peace of mind before signing off on any agreements put forward by one another.
Similarly, corporate mergers and acquisitions are another areas where third-party lawyers can also come into play.
Mergers represent two companies coming together as one entity, which requires careful handling to avoid miscommunications regarding employee rights or other stipulations within internal company policies during such transactions.
Having someone familiar with both sides’ operations objectively look at any deals proposed helps avoid any potential conflict among shareholders following approval from respective boards of directors within each firm being merged as well.
Verdict!
Independent legal advice is invaluable for any situation where two or more parties are involved in a dispute or agreement.
It can provide much-needed guidance to both sides as they look to reach a mutually beneficial resolution while ensuring everyone’s rights and interests are respected throughout the process.
While it may be tempting to cut costs without a third-party lawyer’s opinion, it could lead to more problems that could have been avoided or solved with proper legal counsel from an impartial source.
Therefore, if you are ever in a situation where independent legal advice would be necessary for you and your interests to be best-taken care of, it would be in your best interest to find a qualified good lawyer who can provide the necessary support.
Only then can you ensure that all parties truly get the most favorable deal possible.

How Does SEO Help Your eCommerce Website? Top 5 Benefits
In a market of fierce competition and saturated business ventures, SEO techniques can help increase business revenue, website ranking and traffic drastically. Search Engine Optimization (SEO) can increase your website ranking whenever someone searches for a related topic or niche. This widely accepted method includes […]
BusinessIn a market of fierce competition and saturated business ventures, SEO techniques can help increase business revenue, website ranking and traffic drastically.
Search Engine Optimization (SEO) can increase your website ranking whenever someone searches for a related topic or niche. This widely accepted method includes posting high-value content, using appropriate keywords and making your website easily readable and user-friendly.
However, budding business owners often overlook the major benefits of SEO while creating their website content. For more information, keep reading to find ways in which you may benefit from an e-commerce SEO solution.
Top 5 Benefits Of e-Commerce SEO
1. Maintains Sustainable Traffic
Proper SEO techniques can help drive traffic to your e-commerce website organically. While other promotion methods like social media and paid search engine ads can also generate traffic, they can’t guarantee sustainable traffic in the long run.
But once a potential customer lands on your page, the paid search team exposes them to your ads when they exit. In this way, even if they didn’t make a purchase, the customer is passively reminded that your website offers good value products. This is how SEO can also help remarketing campaigns.
2. Increase Brand Awareness
Besides increasing traffic, SEO can help place your website on the first search results page. This exposure can create awareness of your brand among people who have never heard about it before. If a potential customer chooses a rival site to browse options, it can be a reminder that your brand is a strong alternative.
Being one of the top results can also boost your brand’s credibility and improve visibility.
3. Ensure Quality Traffic
Most visitors to your website may not be potential customers if your content lacks accurate keywords. And short and long-tail keywords help place your website on relevant search engine pages.
For example, if you have a clothing business, only those interested in buying clothes will be directed to you instead of random people on the internet.
4. Develop Customer Goodwill
Every e-commerce business needs to build customer trust so that people keep coming back to purchase from your site. And since there’s no interaction in e-commerce, the process can be more difficult than the traditional physical stores.
However, proper SEO solutions can make potential customers feel at ease while buying from your online store. SEO increases your website rank on web searches since trusted search engines like Google only place verified and legit sites on top.
5. Boost Sales
The previous four benefits all lead to one thing: boosting business sales. A good mix of customer goodwill and high traffic can positively impact your business. However, you must ensure your products remain within a reasonable price range to withstand competition from other brands.
Final Thoughts
Conducting SEO techniques is proven to be the most affordable way of building the popularity of an e-commerce website. However, you can only gain benefits if SEO is done properly since it includes multiple projects focusing on content, web design, user experience, etc.
As such, it may be viable to contact an e-commerce agency that offers these services so that you can derive the maximum benefits.

Is Your Child Charged With A Crime? 3 Things You Must Know About Juvenile Court
So, your child is under police custody because they have committed a crime? That’s a dreaded nightmare for every parent– something so worse that it could lead to a mental breakdown. If something like this happens, you are so overwhelmed by stress that you aren’t […]
Law & LegalSo, your child is under police custody because they have committed a crime?
That’s a dreaded nightmare for every parent– something so worse that it could lead to a mental breakdown. If something like this happens, you are so overwhelmed by stress that you aren’t in a position to think of a solution.
Much like the adult court system, the juvenile court system is complex; however, it is slightly different from the former. And since youth criminal charges aren’t easy to defend, the first thing you must do is consult a criminal lawyer.
After all, as a person with little to no legal knowledge, navigating the system can be quite challenging.
That is why we have put together three things every parent must know about the juvenile court system, so they can be prepared for what lies ahead. Let’s dive in to learn the basics about juvenile court, then!
3 Things You Must Know About Juvenile Court
1. Juvenile Justice Is More About Correcting Behaviors Than Penalizing
The Youth Criminal Justice Act of Canada (YCJA) isn’t focused on penalizing youth for their wrong behaviours. Rather, they correct criminal tendencies through training and therapy, i.e. rehabilitation, unless it’s necessary to remove them from society for their violent behaviour.
Progress reports are submitted in court indicating that the issues behind their odd behaviour are being treated.
2. You Can Hire A Criminal Lawyer To Defend Your Child In A Court Of Law
Much like the adult court system, the juvenile system allows youth to hire an attorney throughout the juvenile process. Therefore, you can connect with a criminal lawyer and discuss your child’s case with them. Since they have been dealing with such cases for years, they will provide you with the best advice and help you navigate the legal system with ease.
However, not every lawyer is competent enough to handle a juvenile criminal case, so always check client testimonials before hiring them.
3. Youth Have The Rights To Counsel
Despite being booked under the Youth Criminal Justice Act of Canada, children are entitled to the right to counsel. As such, it’s the responsibility of the police to inform people about their right to remain silent as soon as they are arrested.
That’s because children are hardly aware of their legal rights and protections that they are entitled to by the Charter. In addition, youth can choose to stay mum when it comes to making any statement until they have a word with their parents.
Summing It Up
No doubt, it’s heartbreaking for every parent to see their children on the wrong side of the law. So, if you’ve been informed about the wrongdoings of your child, consulting a criminal lawyer would be a wise thing to do.
Improper surroundings, lack of education and substance abuse are the top reasons children commit crimes. Hence, parents must keep a watch on the behaviour of their children to make sure they don’t indulge in such heinous activities.
Also, teach them the difference between right and wrong so that they don’t deviate from the right path.
Going through a stressful period because your child is in police custody? Hire a criminal lawyer today!

5 Documents You Need to Prepare for a Divorce Lawyer Consultation
Preparing for a divorce lawyer consultation can be overwhelming. But having the proper documents can make the process smoother and more efficient. This blog post will go over the most important documents you will need to consult a divorce lawyer. How to get a divorce […]
Law & LegalPreparing for a divorce lawyer consultation can be overwhelming. But having the proper documents can make the process smoother and more efficient.
This blog post will go over the most important documents you will need to consult a divorce lawyer.
How to get a divorce lawyer consultation?
First, consider your location. For example, If you live in the Markham area, seek out a professional divorce lawyer in Markham. This way, you can ensure that the lawyer you choose is familiar with the local laws and court system.
Next, do your research. Look for reviews and testimonials from previous clients. It will help you get an idea of the lawyer’s experience and reputation. You can also ask friends and family for recommendations. You can also check with the local bar association for a list of qualified divorce lawyers in your area.
Another important factor to consider is the lawyer’s expertise. Make sure they have experience handling divorce cases similar to yours. it can involve a contested or uncontested divorce or any issues such as child custody or property division.
What Documents are needed for a divorce lawyer consultation?
Here are 5 documents you should gather before your consultation:
Financial Documents
When it comes to divorce, financial matters are often at the forefront. Your lawyer will need to know about your assets, debts, income, and expenses. Gather documents such as:
- Bank statements.
- Credit card statements.
- Pay stubs.
- Tax returns.
- Any other financial records that are relevant to your case.
- Make sure to provide the most recent copies to your lawyer.
Marital Agreement
If you and your spouse have a prenuptial or postnuptial agreement, you must provide a copy to your lawyer. These agreements can significantly impact divorce proceedings. Your lawyer needs to be aware of their existence and terms.
Children-related documents
If you have children, your lawyer will need to know about their living arrangements. Custody arrangements and other relevant information will also be important. Gather documents such as birth certificates, school records. You can also gather any court orders related to your children.
Communication Records
Gather any communication records that are relevant to your case. It can include emails, text messages. It can also include social media messages between you and your spouse.
These records can provide valuable evidence in your case.
Property Records
Gather any records related to your property, such as deeds, mortgages, and other documents that prove ownership. In addition, your lawyer will need to know about any property that needs to be divided during the divorce proceedings.
These documents will ensure that your lawyer has all the information they need. Your lawyer may request additional documents as your case progresses.
Preparing for a divorce lawyer consultation can be a daunting task. By gathering these 5 documents, you can ensure that your lawyer provides you with the best possible representation.
Remember to keep copies of all documents for your records. Be prepared to provide additional documents as your case progresses. With the proper preparation, you can make the divorce process as smooth as possible.

Fitness Advice For Beginners
When you first start working out, it’s easy to become distracted by all the complex training techniques. To avoid becoming overwhelmed, stick to isolation exercises that target just one muscle group at a time. You can always modify the exercises once you see how they […]
Beauty & FashionWhen you first start working out, it’s easy to become distracted by all the complex training techniques. To avoid becoming overwhelmed, stick to isolation exercises that target just one muscle group at a time. You can always modify the exercises once you see how they work out your body. You’ll eventually master the technique and feel confident enough to move on to more advanced exercises. Here are some helpful fitness advice for beginners:
Begin by doing something you enjoy. Don’t try to do anything too strenuous at first, because you’ll end up hurting yourself and losing motivation. Start small and increase your distance gradually. Make sure to fuel your body properly by incorporating exercises into your daily routine. Make sure you take breaks during the workouts to recharge. And, remember that it’s not a race, so make sure you enjoy the workouts!
Henry Cavill is another Hollywood superstar who offers great fitness advice for beginners. The busy actor has reportedly taken to the gym for various roles, including his role as Superman. He says that every body is different and that you should do what works for you. While he likes cardio exercises to tone his glute and hamstrings, he encourages beginners to do it their way, too. While Henry Cavill has a busy schedule, he has found the time to work out, and he’s sharing his tips on YouTube.
Another piece of fitness advice for beginners is to set a routine. Don’t work out too often. Instead, opt for shorter but more frequent workout sessions. The aerobic benefits of short workouts can make them more convenient to fit into your busy schedule. It’s also important to take time to rest and recover after your workouts. After all, any activity is better than doing nothing at all. Moreover, you can add activities you enjoy to your routine.
Starting a new fitness regimen can be intimidating, but it’s crucial to start your new journey on the right foot. To stay motivated, set small goals. Break up your ultimate goal and set smaller goals. Remember that results come from consistency and regular activity. Don’t let yourself get overwhelmed by short-term goals. Keep in mind that your goal will be attainable if you keep on with it. If you follow these tips, you’ll see results very soon!
Working out isn’t always easy, but you may want to stick with it if you enjoy it. Once you find a routine that you enjoy, you might want to make it a regular part of your life. However, getting started can be intimidating, and you might not know what to do first. Finding the right exercise for you is crucial, and it may take some time to find the right workout for you. Once you’ve found the one that suits your body type, the rest will follow.
The key to sticking with your exercise program is to make it part of your daily routine. Start small, by adding one or two workouts to your calendar each week. You can also include exercise breaks to watch TV or read a book. Just make sure to include a variety of activities, from running to swimming to yoga to swimming. Exercise doesn’t have to take up a lot of your time; a 20-minute brisk walk during your lunch hour to a strength training session can be effective.





